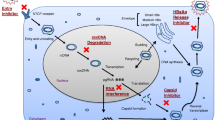
Abstract
The ability to culture hepatitis C virus (HCV) and the determination of the three-dimensional structure of the replication elements of the HCV genome allowed the rapid development of antiviral molecules directly targeting specific enzymes that allow HCV to reproduce itself. This new class of drugs, known as specifically targeted antiviral therapy for HCV (STAT-C), offers a new paradigm for the treatment of this elusive virus. During recent years, multiple STAT-C agents were introduced into clinical trials, but only a few have overcome the hurdles often associated with early clinical drug development. This article discusses the latest clinical trial findings on the emerging STAT-C agents for the treatment of HCV, particularly the agents that have progressed to phase 2 and phase 3 in clinical trials.
Similar content being viewed by others
References and Recommended Reading
Fried MW, Shiffman ML, Reddy R, et al.: Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002, 347:975–982.
Manns MP, McHutchison JG, Gordon SC, et al.: the International Hepatitis Interventional Therapy Group: Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet 2001, 358:958–965.
Muir AJ, Bornstein JD, Killenberg PG: Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med 2004, 350:2265–2271.
Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al.: Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004, 351:438–450.
Jeffers LJ, Cassidy W, Howell CD, et al.: Peginterferon alfa-2a (40 kd) and ribavirin for black African American patients with chronic HCV genotype 1. Hepatology 2004, 39:1702–1708.
Shiffman ML, Di Bisceglie AM, Lindsay KL, et al.: Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis Trial Group: Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology 2004, 126:1015–1023.
Marcellin P, Freilich B, Andreone P, et al.: HCV-RNA status at week 12 of treatment with peginterferon alfa-2a/RBV predicts SVR in patients with prior non-response to pegylated interferon alfa-2b/RBV: results from REPEAT study. J Hepatol 2008, 48(Suppl 2):S301.
Gramenzi A, Andreone P, Cursaro C, et al.: A randomized trial of induction doses of interferon alone or in combination with ribavirin or ribavirin plus amantadine for treatment of nonresponder patients with chronic hepatitis C. J Gastroenterol 2007, 42:362–367.
Backus LI, Boothroyd DB, Phillips BR, Mole LA: Predictors of response of U.S. veterans to treatment for the hepatitis C virus. Hepatology 2007, 46:37–47.
Zeuzem S, Hezode C, Ferenci P, et al.: Telaprevir in combination with peginterferon alpha-2a with or without ribavirin in the treatment of chronic hepatitis C: final results of the PROVE2 study [abstract 243]. Presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases. San Francisco, CA; October 31–November 4, 2008.
Kwo P, Lawitze E, McCone J, et al.: Boceprevir plus peginterferon alfa-2b/ribavirin for the treatment of genotype 1 chronic hepatitis C in previously untreated patients; interim results from HCV SPRINT-1 study [abstract LB16]. Presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases. San Francisco, CA; October 31–November 4, 2008.
Lalezari J, Gane E, Rodriguez-Torres M, et al.: Potent antiviral activity of the HCV nucleoside polymerase inhibitor R7128 with PEG-IFN and ribavirin: interim results of R7128 500 mg bid for 28 days [abstract 66]. Presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases. San Francisco, CA; October 31–November 4, 2008.
Rodriguez-Torres M, Lalezaei J, Gane E, et al.: Potent antiviral response to the HCV nucleoside polymerase inhibitor R7128 for 28 days with peg-IFN and ribavirin: subanalysis by race/ethnicity, weight, and HCV genotype [abstract 1899]. Presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases. San Francisco, CA; October 31–November 4, 2008.
Gane EJ, Rodriguez-Torres M, Nelson DR, et al.: Antiviral activity of the HCV nucleoside polymerase inhibitor R7128 in HCV genotype 2 and 3 prior nonresponders: results of R7128 1500 mg BID with Peg-IFN and ribavirin for 28 days [abstract LB10]. Presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases. San Francisco, CA; October 31–November 4, 2008.
McHutchinson JG, Shiffman ML, Terrault N, et al.: A phase 2b study of telaprevir with peginterferon alpha 2a and ribavirin in hepatitis C genotype 1 null and partial responders and relapsers following a prior course of peginterferon alpha 2a/b and ribavirin therapy: PROVE3 interim results [abstract 269]. Presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases. San Francisco, CA; October 31–November 4, 2008.
Shiffman ML, Berg T, Poordad F, et al.: A study of telaprevir combined with peginterferon alfa-2a and ribavirin in patients with well documented non-response or relapse after previous peginterferon alfa-2a and ribavirin treatment: interim analysis [PROVE abstract 1852]. Presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases. San Francisco, CA; October 31–November 4, 2008.
Nettles R, Chien C, Chung E, et al.: BMS-790052 is a first in class potent hepatitis C virus (HCV) NS5A inhibitor for patients with chronic HCV infection: results from a proof-of-concept study [abstract LB12]. Presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases. San Francisco, CA; October 31–November 4, 2008.
Rossignol J, Elfert A, El-Gohary Y, et al.: Interim data from a randomized controlled trial of nitazoxanide-peginterferon-ribavirin, nitazoxanide-peginterferon and peginterferon-ribavirin in the treatment of patients with chronic hepatitis C genotype 4 [abstract 178]. Presented at the 58th Annual Meeting of the American Association for the Study of Liver Diseases. Boston, MA; November 2–6, 2007.
Watashi K, Ishii N, Hijikata M, et al.: Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol Cell 2005, 19:111–122.
Pockros PJ, Nelson D, Godofsky E, et al.: R1626 plus peginterferon alfa-2a provides potent suppression of hepatitis C virus RNA and significant antiviral synergy in combination with ribavirin. Hepatology 2008, 48:385–397.
Kieffer TL, Sarrazin C, Miller JS, et al.: Telaprevir and pegylated interferon-alpha-2a inhibit wild-type and resistant genotype 1 hepatitis C virus replication in patients. Hepatology 2007, 47:631–639.
Sarrazin C, Kieffer TL, Bartels D, et al.: Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with protease inhibitor telaprevir. Gastroenterology 2007, 132:1767–1777.
Kuntzen T, Timm J, Berical A, et al.: Naturally occurring dominant resistance mutations to hepatitis C virus protease and polymerase inhibitors in treatment-naïve patients. Hepatology 2008, 48:1769–1778.
Sulkowski MS, McHutchinson JG: Interim analysis and viral variant evaluation from a phase 2 study of telaprevir with peginterferon alfa-2A and ribavirin in treatment-naïve subjects with hepatitis C [abstract V1383]. Presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy. Chicago, IL; September 17–20, 2007.
Forns X, Marcellin P, Goeser T, et al.: Phase 2 study of telaprevir administered q8h or q12h with peginterferon alfa-2a or alfa-2b and ribavirin in treatment-naïve patients with genotype 1 hepatitis C: week 12 interim results [abstract 1854]. Presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases. San Francisco, CA; October 31–November 4, 2008.
Grünberger C, Wyles DL, Kaihara KA, Schooley RT: Threedrug synergistic interactions of small molecular inhibitors of hepatitis C virus replication. J Infect Dis 2008, 197:42–45.
Tan H, Rajyaguru S, Wu T, et al.: Combination of the NS3/4A protease inhibitor ITMN-191 [7227] with the active moiety of NS5B inhibitors R1626 or R7128 enhances replicon clearance and reduces emergence of drug resistant variants [abstract 1885]. Presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases. San Francisco, CA; October 31–November 4, 2008.
Olsen D, et al.: A combination of direct antiviral compounds show synergistic activity in vitro and enhanced efficacy in vivo [abstract FP149]. Presented at the 18th Conference of the Asian Pacific Association for the Study of the Liver. Seoul, South Korea; March 23–26, 2008.
Roche, InterMune, and Pharmasset announce initiation of INFORM-1, the first dual-combination clinical trial with oral antivirals in hepatitis C [press release]. Available at http://www.hivandhepatitis.net/2008icr/aasld/docs/11408. Accessed November 10, 2008.
Sherman KE, Fleischer R, Laessig K, et al.: FDA Antiviral Products Advisory Committee: Development of novel agents for the treatment of chronic hepatitis C infection: summary of the FDA Antiviral Products Advisory Committee recommendations. Hepatology 2007, 46:2014–2020.
US National Institutes of Health. Clinical Trials.gov Web site. A phase 3 study of two dose regimens of telaprevir in combination with peginterferon alfa-2a (Pegasys) and ribavirin (Copegus) in treatment naïve subjects with genotype 1 chronic hepatitis C. Federal identifier NCT00627926. http://www.clinicaltrials.gov/ct2/show/NCT00627926?term=telaprevir&phase=2&rank=1. Accessed August 2008.
US National Institutes of Health. Clinical Trials.gov Web site. A randomized, double-blind, placebo-controlled, phase 3 trial of two regimens of telaprevir (with and without delayed start) combine with pegylated interferon alfa-2a (Pegasys) and ribavirin (Copegus) in subjects with chronic, genotype 1, hepatitis C infection who failed prior standard treatment. Federal identifier NCT00703118. Accessed August 2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morelli, G., Nelson, D.R. STAT-C: New therapies cannot get here fast enough. Curr hepatitis rep 8, 73–80 (2009). https://doi.org/10.1007/s11901-009-0011-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-009-0011-0