Abstract
Purpose of Review
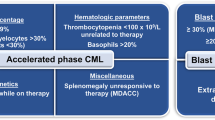
While most patients with chronic myeloid leukemia (CML) present in a chronic phase and are expected to have a normal life expectancy, some patients present with or progress to a more aggressive accelerated phase (AP) or blast phase (BP) of CML. Herein, we discuss the diagnostic considerations of advanced phase CML and review its contemporary management.
Recent Findings
Later-generation, more potent BCR::ABL1 tyrosine kinase inhibitors (TKIs) such as ponatinib may result in superior outcomes in patients with advanced phase CML. For CML-BP, combination approaches directed against the blast immunophenotype appear superior to TKI monotherapy. The role of allogeneic stem cell transplantation is controversial in CML-AP but has consistently been shown to improve outcomes for patients with CML-BP.
Summary
Advanced phase CML, particularly CML-BP, remains a poor risk subtype of CML. However, novel combination approaches using later-generation TKIs are being explored in clinical trials and may lead to improved outcomes.

Similar content being viewed by others
Abbreviations
- CML:
-
Chronic myeloid leukemia
- ELN:
-
European LeukemiaNet
- WHO:
-
World Health Organization
- BM:
-
Bone marrow
- WBC:
-
White blood cells
- ACA:
-
Additional cytogenetic abnormality
- PB:
-
Peripheral blood
- CNS:
-
Central nervous system
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TML. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34:2851–7.
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020;95:691–709.
Sasaki K, Strom SS, O’Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2:e186–93.
Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016;34:2333–40.
Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54.
Hoffmann VS, Baccarani M, Hasford J, et al. Treatment and outcome of 2904 CML patients from the EUTOS population-based registry. Leukemia. 2017;31:593–601.
O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004.
• Jain P, Kantarjian HM, Ghorab A, et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: cohort study of 477 patients. Cancer. 2017;123:4391–402. Retrospective analysis identifying prognostic factors in patients with CML-BP.
Senapati J, Jabbour E, Kantarjian H, Short NJ. Pathogenesis and management of accelerated and blast phases of chronic myeloid leukemia. Leukemia. 2023;37:5–17.
Giles FJ, Cortes JE, Kantarjian HM, O’Brien SM. Accelerated and blastic phases of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18:753–74.
•• Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84. The European LeukemiaNet recommendations on CML, which include definitions of CML-AP and CML-BP.
•• Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19. The WHO 2020 recommendations that removed the category of CML-AP and replaced it with “high-risk CML.
Wang W, Chen Z, Hu Z, et al. Clinical significance of trisomy 8 that emerges during therapy in chronic myeloid leukemia. Blood Cancer J. 2016;6:e490-e.
Issa GC, Kantarjian HM, Gonzalez GN, et al. Clonal chromosomal abnormalities appearing in Philadelphia chromosome–negative metaphases during CML treatment. Blood. 2017;130:2084–91.
Senapati J, Sasaki K. Chromosomal instability in chronic myeloid leukemia: mechanistic insights and effects. Cancers. 2022;14:2533.
Clark RE, Apperley JF, Copland M, Cicconi S. Additional chromosomal abnormalities at chronic myeloid leukemia diagnosis predict an increased risk of progression. Blood Adv. 2021;5:1102–9.
Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–27.
Bataller A, Sasaki K, Jabbour E, et al. Prognostic model of transformation to blast phase in patients with chronic myeloid leukemia. Blood. 2022;140:3895–6.
Hehlmann R, Voskanyan A, Lauseker M, et al. High-risk additional chromosomal abnormalities at low blast counts herald death by CML. Leukemia. 2020;34:2074–86.
Wang W, Cortes JE, Lin P, et al. Clinical and prognostic significance of 3q26.2 and other chromosome 3 abnormalities in CML in the era of tyrosine kinase inhibitors. Blood. 2015;126:1699–706.
Wang W, Cortes JE, Tang G, et al. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016;127:2742–50.
Braun TP, Eide CA, Druker BJ. Response and resistance to BCR-ABL1-targeted therapies. Cancer Cell. 2020;37:530–42.
Carella AM, Garuti A, Cirmena G, et al. Kinase domain mutations of BCR-ABL identified at diagnosis before imatinib-based therapy are associated with progression in patients with high Sokal risk chronic phase chronic myeloid leukemia. Leuk Lymphoma. 2010;51:275–8.
Loy K, Zenger M, Meggendorfer M, et al. Analysis of mechanisms of blast crisis in chronic myeloid leukemia by whole genome sequencing. Blood. 2020;136:19.
Machnicki MM, Pepek M, Solarska I, et al. ASXL1 mutations detectable at diagnosis may predict response to imatinib in patients with chronic myeloid leukemia. Blood. 2019;134:4148.
Marum JE, Yeung DT, Purins L, et al. ASXL1 and BIM germ line variants predict response and identify CML patients with the greatest risk of imatinib failure. Blood Adv. 2017;1:1369–81.
Menezes J, Salgado RN, Acquadro F, et al. ASXL1, TP53 and IKZF3 mutations are present in the chronic phase and blast crisis of chronic myeloid leukemia. Blood Cancer J. 2013;3:e157-e.
Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–9.
Branford S, Wang P, Yeung DT, et al. Integrative genomic analysis reveals cancer-associated mutations at diagnosis of CML in patients with high-risk disease. Blood. 2018;132:948–61.
Lee KL, Ko TK, Saw NYL, et al. Validation and refinement of a RUNX1 mutation-associated gene expression signature in blast crisis chronic myeloid leukemia. Leukemia. 2022;36:892–6.
Adnan-Awad S, Kim D, Hohtari H, et al. Characterization of p190-Bcr-Abl chronic myeloid leukemia reveals specific signaling pathways and therapeutic targets. Leukemia. 2021;35:1964–75.
Verma D, Kantarjian HM, Jones D, et al. Chronic myeloid leukemia (CML) with P190BCR-ABL: analysis of characteristics, outcomes, and prognostic significance. Blood. 2009;114:2232–5.
Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117:3733–6.
Hehlmann R, Lauseker M, Saußele S, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–406.
Lauseker M, Hasford J, Saussele S, et al. Smokers with chronic myeloid leukemia are at a higher risk of disease progression and premature death. Cancer. 2017;123:2467–71.
Ohanian M, Kantarjian HM, Shoukier M, et al. The clinical impact of time to response in de novo accelerated-phase chronic myeloid leukemia. Am J Hematol. 2020;95:1127–34.
Ohanian M, Kantarjian HM, Quintas-Cardama A, et al. Tyrosine kinase inhibitors as initial therapy for patients with chronic myeloid leukemia in accelerated phase. Clin Lymphoma Myeloma Leuk. 2014;14:155-62.e1.
Cortes JE. A second-generation TKI should always be used as initial therapy for CML. Blood Adv. 2018;2:3653–5.
Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231–7.
NCCN. Chronic Myeloid leukemia NCCN website: NCCN Version 3.2022. https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf. 2022. Accessed 1 May 2023.
Apperley JF, Cortes JE, Kim D-W, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START A trial. J Clin Oncol. 2009;27:3472–9.
Francesca P, Fausto C, Giuliana A, et al. The long-term durability of cytogenetic responses in patients with accelerated phase chronic myeloid leukemia treated with imatinib 600 mg: the GIMEMA CML Working Party experience after a 7-year follow-up. Haematologica. 2009;94:205–12.
le Coutre PD, Giles FJ, Hochhaus A, et al. Nilotinib in patients with Ph+ chronic myeloid leukemia in accelerated phase following imatinib resistance or intolerance: 24-month follow-up results. Leukemia. 2012;26:1189–94.
Ottmann O, Saglio G, Apperley JF, et al. Long-term efficacy and safety of dasatinib in patients with chronic myeloid leukemia in accelerated phase who are resistant to or intolerant of imatinib. Blood Cancer J. 2018;8:88.
•• Senapati J, Sasaki K, Issa GC, et al. Management of chronic myeloid leukemia in 2023 – common ground and common sense. Blood Cancer J. 2023;13:58. Prospective study of ponatinib monotherapy across different CML subgroups, including CML-AP and CML-BP.
O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–12.
Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome–positive leukemias. N Engl J Med. 2013;369:1783–96.
Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome–positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404.
Cortes J, Rousselot P, Kim D-W, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2006;109:3207–13.
Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–42.
Giles FJ, Kantarjian HM, le Coutre PD, et al. Nilotinib is effective in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blastic phase. Leukemia. 2012;26:959–62.
• Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study. Cancer. 2010;116:3852–61. Retrospective analysis of patients with CML-MBP, showing the benefit of combination therapies with chemotherapy or a hypomethylating agent plus a TKI, and also the benefit of HSCT.
• Cortes J, Apperley J, Lomaia E, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138:2042–50. Prospective study evaluating FLAG-Ida plus ponatinib in patients with CML-BP, most of whom had myeloid blast phase.
Saxena K, Jabbour E, Issa G, et al. Impact of frontline treatment approach on outcomes of myeloid blast phase CML. J Hematol Oncol. 2021;14:94.
Copland M, Slade D, McIlroy G, et al. Ponatinib with fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor chemotherapy for patients with blast-phase chronic myeloid leukaemia (MATCHPOINT): a single-arm, multicentre, phase 1/2 trial. Lancet Haematol. 2022;9:e121–32.
Abaza Y, Kantarjian H, Alwash Y, et al. Phase I/II study of dasatinib in combination with decitabine in patients with accelerated or blast phase chronic myeloid leukemia. Am J Hematol. 2020;95:1288–95.
Maiti A, Franquiz Miguel J, Ravandi F, et al. Venetoclax and BCR-ABL tyrosine kinase inhibitor combinations: outcome in patients with Philadelphia chromosome-positive advanced myeloid leukemias. Acta Haematol. 2021;143:567–73.
Senapati J, Ravandi F, Dinardo CD, et al. A phase 2 study of the combination of decitabine (DAC), venetoclax (VEN), and ponatinib in patients (Pts) with chronic myeloid leukemia (CML) in accelerated phase (AP)/myeloid blast phase (MBP) or Philadelphia-chromosome positive (Ph+) acute myeloid leukemia (AML). Journal of Clinical Oncology 2023;41.
Strati P, Kantarjian H, Thomas D, et al. HCVAD plus imatinib or dasatinib in lymphoid blastic phase chronic myeloid leukemia. Cancer. 2014;120:373–80.
Morita K, Kantarjian HM, Sasaki K, et al. Outcome of patients with chronic myeloid leukemia in lymphoid blastic phase and Philadelphia chromosome–positive acute lymphoblastic leukemia treated with hyper-CVAD and dasatinib. Cancer. 2021;127:2641–7.
Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–47.
Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375:740–53.
Jabbour E, Short NJ, Jain N, et al. Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: a US, single-centre, single-arm, phase 2 trial. Lancet Haematol. 2023;10:e24–34.
Nguyen D, Jabbour E, Short N, et al. A phase II study of the sequential combination of low-intensity chemotherapy (mini-hyper-CVD) and ponatinib followed by blinatumomab and ponatinib in patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). Blood. 2022;140:6127–9.
Hu B, Lin X, Lee HC, et al. Timing of allogeneic hematopoietic cell transplantation (alloHCT) for chronic myeloid leukemia (CML) patients. Leuk Lymphoma. 2020;61:2811–20.
• Radujkovic A, Dietrich S, Blok H-J, et al. Allogeneic stem cell transplantation for blast crisis chronic myeloid leukemia in the era of tyrosine kinase inhibitors: a retrospective study by the EBMT Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2019;25:2008–16. Study showing the benefit of HSCT in patients with T315I-mutated CML-BP, even when treated with ponatinib.
Barrett AJ, Ito S. The role of stem cell transplantation for chronic myelogenous leukemia in the 21st century. Blood. 2015;125:3230–5.
Nicolini FE, Basak GW, Kim D-W, et al. Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer. 2017;123:2875–80.
Kotrová M, Koopmann J, Trautmann H, et al. Prognostic value of low-level MRD in adult acute lymphoblastic leukemia detected by low- and high-throughput methods. Blood Adv. 2022;6:3006–10.
Short NJ, Jabbour E, Macaron W, et al. Ultrasensitive NGS MRD assessment in Ph+ ALL: prognostic impact and correlation with RT-PCR for BCR::ABL1. Am J Hematol. 2023;98(8):1196–203.
Short NJ, Kantarjian H, Ravandi F, et al. High-sensitivity next-generation sequencing MRD assessment in ALL identifies patients at very low risk of relapse. Blood Adv. 2022;6:4006–14.
DeFilipp Z, Ancheta R, Liu Y, et al. Maintenance tyrosine kinase inhibitors following allogeneic hematopoietic stem cell transplantation for chronic myelogenous leukemia: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2020;26:472–9.
Funding
Supported by an MD Anderson Cancer Center Support Grant (CA016672) and SPORE. N. J. S. is supported by the American Society of Hematology Junior Faculty Scholar Award in Clinical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
N. J. S. has served as consultant for Pfizer Inc., GSK, NKARTA, and Sanofi, reports receiving research grants from Takeda Oncology, Astellas Pharma Inc., Xencor, Stemline Therapeutics, and NextCure, and has received honoraria from Novartis, Amgen, Pfizer Inc., Astellas Pharma Inc., Sanofi, and BeiGene. J. S. has been on the Advisory Board of Kite. E. J.: AbbVie: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Short, N.J., Senapati, J. & Jabbour, E. An Update on the Management of Advanced Phase Chronic Myeloid Leukemia. Curr Hematol Malig Rep 18, 234–242 (2023). https://doi.org/10.1007/s11899-023-00709-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-023-00709-4