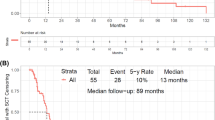
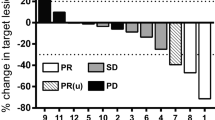
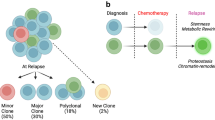
Abstract
Purpose of Review
This review will discuss the data and controversies related to HCT in the front-line and relapsed/refractory setting in the context of newly available targeted immunotherapies.
Recent Findings
Recent studies in adult Ph-negative ALL support the use of measurable residual disease (MRD) response to front-line therapy to guide consolidation. As such, most MRD-negative patients do not require front-line HCT. Blinatumomab benefits patients with B-ALL with MRD+ complete response (CR) and can be used as a bridge to HCT; whether HCT is still required in this setting is an area of ongoing inquiry. Blinatumomab and inotuzumab result in high rates of MRD negative CR in adults with relapsed/refractory ALL and allow more patients with relapsed disease to receive HCT. Chimeric antigen receptor T cell (CAR-T) therapies may serve as a bridge to HCT or as a stand-alone therapy for relapsed/refractory patients; data suggests there may be greater benefit to consolidating CAR-T with HCT in HCT-naïve adults.
Summary
The decision to incorporate consolidative allogeneic HCT into front-line therapy should be primarily guided by MRD status and the ALL regimen utilized. Targeted immunotherapies result in high MRD-negative CR rates, allowing more adults with relapsed/refractory ALL to be successfully bridged to HCT; early incorporation of these therapies may also prove valuable in reducing the need for HCT in the front-line setting by increasing MRD negative CR rates.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rowe JM, Buck G, Burnett AK, ECOG, MRC/NCRI Adult Leukemia Working Party, Chopra R, Wiernik PH, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106(12):3760–7.
• Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827–33. This is a landmark study in adult ALL that initially demonstrated the benefit of allogeneic HCT as consolidation for younger adults with ALL.
Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–60.
Matsumura T, Kami M, Yamaguchi T, Yuji K, Kusumi E, Taniguchi S, et al. Allogeneic cord blood transplantation for adult acute lymphoblastic leukemia: retrospective survey involving 256 patients in Japan. Leukemia. 2012;26(7):1482–6.
Srour SA, Milton DR, Bashey A, Karduss-Urueta A, al Malki MM, Romee R, et al. Haploidentical transplantation with post-transplantation cyclophosphamide for high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2017;23(2):318–24.
Han LJ, Wang Y, Fan ZP, Huang F, Zhou J, Fu YW, et al. Haploidentical transplantation compared with matched sibling and unrelated donor transplantation for adults with standard-risk acute lymphoblastic leukaemia in first complete remission. Br J Haematol. 2017;179(1):120–30.
• Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548–59. This this the results of the prospective C10403 clinical trial demonstrating improving results for young adults treated with pediatric ALL therapy.
DeAngelo DJ, Stevenson KE, Dahlberg SE, Silverman LB, Couban S, Supko JG, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29(3):526–34.
Short NJ, Jabbour E, Albitar M, de Lima M, Gore L, Jorgensen J, et al. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: a consensus of North American experts. Am J Hematol. 2019;94(2):257–65.
Giebel S, Marks DI, Boissel N, Baron F, Chiaretti S, Ciceri F, et al. Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: a position statement of the European working Group for Adult Acute Lymphoblastic Leukemia (EWALL) and the acute leukemia working Party of the European Society for Blood and Marrow transplantation (EBMT). Bone Marrow Transplant. 2019;54(6):798–809.
DeFilipp Z, Advani AS, Bachanova V, Cassaday RD, Deangelo DJ, Kebriaei P, et al. Hematopoietic cell transplantation in the treatment of adult acute lymphoblastic leukemia: updated 2019 evidence-based review from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019;25(11):2113–23.
• Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580. This meta-analysis demonstrates the prognostic significance of MRD throughout the treatment course in both pediatric and adult ALL.
Gokbuget N, Kneba M, Raff T, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868–76.
Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116–23.
Holowiecki J, Krawczyk-Kulis M, Giebel S, Jagoda K, Stella-Holowiecka B, Piatkowska-Jakubas B, et al. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The polish adult leukemia group ALL 4-2002 MRD study. Br J Haematol. 2008;142(2):227–37.
Ribera JM, Oriol A, Morgades M, Montesinos P, Sarrà J, González-Campos J, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32(15):1595–604.
Jain N, Lamb AV, O'Brien S, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood. 2016;127(15):1863–9.
El Fakih R, Savani B, Mohty M, et al. Hematopoietic cell transplant consideration for Philadelphia chromosome-like acute lymphoblastic leukemia patients. Biol Blood Marrow Transplant. 2020;26(1):e16–20.
Seftel MD, Neuberg D, Zhang MJ, Wang HL, Ballen KK, Bergeron J, et al. Pediatric-inspired therapy compared to allografting for Philadelphia chromosome-negative adult ALL in first complete remission. Am J Hematol. 2016;91(3):322–9.
Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J. 2011;1(4):e16.
Logan AC, Vashi N, Faham M, Carlton V, Kong K, Buño I, et al. Immunoglobulin and T cell receptor gene high-throughput sequencing quantifies minimal residual disease in acute lymphoblastic leukemia and predicts post-transplantation relapse and survival. Biol Blood Marrow Transplant. 2014;20(9):1307–13.
• Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–31. This prospective trial demonstrated high response rates of blinatumomab for B-ALL patients with MRD and led to the approval of this agent for MRD+ disease.
Richard-Carpentier G, Kantarjian H, Short N, et al. Updated results from the phase II study of hyper-CVAD in sequential combination with blinatumomab in newly diagnosed adults with B-cell acute lymphoblastic leukemia (B-ALL). Blood. 2019;134(supplement):3807.
Fleming S, Venn N, Reynolds J, et al. Preliminary minimal residual disease analysis of the australasian leukaemia & lymphoma group (ALLG) ALL8 study of front-line blinatumomab with chemotherapy in adults with ph negative B-cell acute lymphoblastic leukaemia. Blood. 2019;134(supplement):1300.
Kantarjian H, Ravandi F, Short NJ, Huang X, Jain N, Sasaki K, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2018;19(2):240–8.
Jabbour EJ, Sasaki K, Ravandi F, Short NJ, Garcia-Manero G, Daver N, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy (mini-HCVD) with or without blinatumomab versus standard intensive chemotherapy (HCVAD) as frontline therapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: a propensity score analysis. Cancer. 2019;125(15):2579–86.
Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–50.
Topp MS, Gokbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–40.
Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66.
Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47.
Jabbour EJ, Gokbuget N, Kantarjian HM, et al. Transplantation in adults with relapsed/refractory acute lymphoblastic leukemia who are treated with blinatumomab from a phase 3 study. Cancer. 2019;125(23):4181–92.
Yoon JH, Min GJ, Park SS, Park S, Lee SE, Cho BS, et al. Feasible outcome of blinatumomab followed by allogeneic hematopoietic cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer Med. 2019;8(18):7650–9.
Keating AK, Gossai N, Phillips CL, Maloney K, Campbell K, Doan A, et al. Reducing minimal residual disease with blinatumomab prior to HCT for pediatric patients with acute lymphoblastic leukemia. Blood Adv. 2019;3(13):1926–9.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic LEUKEMIA. N Engl J Med. 2016;375(8):740–53.
Marks DI, Kebriaei P, Stelljes M, Gökbuget N, Kantarjian H, Advani AS, et al. Outcomes of allogeneic stem cell transplantation after inotuzumab ozogamicin treatment for relapsed or refractory acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2019;25(9):1720–9.
Kantarjian HM, DeAngelo DJ, Stelljes M, Liedtke M, Stock W, Gökbuget N, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474–87.
Jabbour EJ, DeAngelo DJ, Stelljes M, Stock W, Liedtke M, Gökbuget N, et al. Efficacy and safety analysis by age cohort of inotuzumab ozogamicin in patients with relapsed or refractory acute lymphoblastic leukemia enrolled in INO-VATE. Cancer. 2018;124(8):1722–32.
Kebriaei P, Cutler C, de Lima M, et al. Management of important adverse events associated with inotuzumab ozogamicin: expert panel review. Bone Marrow Transplant. 2018;53(4):449–56.
Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48.
Zhang Y, Chen H, Song Y, et al. Post-chimeric antigen receptor T-cell therapy haematopoietic stem cell transplantation for 52 cases with refractory/relapsed B-cell acute lymphoblastic leukaemia. Br J Haematol. 2019. https://doi.org/10.1111/bjh.16339.
Frey NV, Shaw PA, Hexner EO, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol. 2019. https://doi.org/10.1200/JCO.19.01892:JCO1901892.
Park JH, Riviere I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–59.
Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652–63.
Lin JK, Lerman BJ, Barnes JI, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin Oncol. 2018. https://doi.org/10.1200/JCO.2018.79.0642:JCO2018790642.
Xian Z, Yang J, Song DJ, et al. Analysis of factors predicting response of 254 patients received CD19 CAR-T cell therapy for relapsed/refractory (R/R) B-ALL. Blood. 2019;Supplement.
Shadman M, Gauthier J, Hay KA, Voutsinas JM, Milano F, Li A, et al. Safety of allogeneic hematopoietic cell transplant in adults after CD19-targeted CAR T-cell therapy. Blood Adv. 2019;3(20):3062–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
IY, JC, and LM declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stem Cell Transplantation
Rights and permissions
About this article
Cite this article
Yurkiewicz, I., Craig, J. & Muffly, L. Hematopoietic Cell Transplantation for Philadelphia Chromosome Negative Adult Acute Lymphoblastic Leukemia in the Modern Era of Immune Therapy. Curr Hematol Malig Rep 15, 187–193 (2020). https://doi.org/10.1007/s11899-020-00579-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-020-00579-0