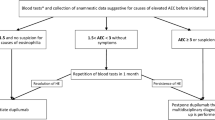
Abstract
Purpose of Review
Hypereosinophilic syndrome (HES) is characterized by persistent hypereosinophilia associated with end-organ damage. As our understanding of the pathogenesis of various forms of HES broadens, so does our ability to tailor steroid-sparing therapies for each subtype. The purpose of this review is to summarize recent literature related to the etiology, diagnosis, and management of HES.
Recent Findings
Mutations involved in subsets of HES can guide the choice of tyrosine kinase inhibitors beyond just imatinib. Several biologics that target interleukin-5 or its receptor have shown beneficial and selective eosinophil-reducing effects in clinical trials for asthma and other disorders including HES. Early clinical data with emerging therapies such as dexpramipexole and anti-Siglec-8 antibody show promise, but need to be confirmed in randomized trials.
Summary
Several new biologics and tyrosine kinase inhibitors have been shown to lower eosinophil numbers, but more randomized trials are needed to confirm efficacy in HES.

Similar content being viewed by others
Abbreviations
- AEC:
-
Absolute eosinophil count
- ASXL1:
-
Putative polycomb group protein ASXL1
- CBL:
-
Cbl ubiquitin-protein ligase
- CD:
-
Cluster of differentiation
- EGID:
-
Eosinophilic gastrointestinal disease
- EGPA:
-
Eosinophilic granulomatosis with polyangiitis
- EMR1:
-
EGF-like module containing mucin-like hormone receptor
- EoE:
-
Eosinophilic esophagitis
- EZH2:
-
Enhancer of zeste homolog 2
- GC:
-
Glucocorticoid
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- HE:
-
Hypereosinophilia
- HES:
-
Hypereosinophilic syndrome
- IL:
-
Interleukin
- JAK:
-
Janus kinase
- NOTCH1:
-
Notch homolog 1, translocation-associated
- STAT:
-
Signal transducer and activator of transcription
- SETBP1:
-
SET-binding protein 1
- sq:
-
Subcutaneous
- TET2:
-
Tet methylcytosine dioxygenase 2
- TSLP:
-
Thymic stromal lymphopoietin
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Bochner B. The eosinophil: for better or worse, in sickness and in health. Ann Allergy Asthma Immunol. 2018; (in press).
Diny NL, Rose NR, Cihakova D. Eosinophils in autoimmune diseases. Front Immunol. 2017;8:484.
Valent P, Gleich GJ, Reiter A, Roufosse F, Weller PF, Hellmann A, et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev Hematol. 2012;5(2):157–76.
Walker S, Wang C, Walradt T, Hong BS, Tanner JR, Levinsohn JL, et al. Identification of a gain-of-function STAT3 mutation (p.Y640F) in lymphocytic variant hypereosinophilic syndrome. Blood. 2016;127(7):948–51.
Lin AY, Nutman TB, Kaslow D, Mulvihill JJ, Fontaine L, White BJ, et al. Familial eosinophilia: clinical and laboratory results on a U.S. kindred. Am J Med Genet. 1998;76(3):229–37.
• Prakash Babu S, Chen YK, Bonne-Annee S, Yang J, Maric I, Myers TG, et al. Dysregulation of interleukin 5 expression in familial eosinophilia. Allergy. 2017;72(9):1338–45. This study showed that IL-5 and IL-5R dysregulation are involved in the pathogenesis of familial eosinophilia in one family cohort.
Rioux JD, Stone VA, Daly MJ, Cargill M, Green T, Nguyen H, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63(4):1086–94.
Del Bel KL, Ragotte RJ, Saferali A, Lee S, Vercauteren SM, Mostafavi SA, et al. JAK1 gain-of-function causes an autosomal dominant immune dysregulatory and hypereosinophilic syndrome. J Allergy Clin Immunol. 2017;139(6):2016–20.
Wang SA, Hasserjian RP, Tam W, Tsai AG, Geyer JT, George TI, et al. Bone marrow morphology is a strong discriminator between chronic eosinophilic leukemia, not otherwise specified and reactive idiopathic hypereosinophilic syndrome. Haematologica. 2017;102(8):1352–60.
• Wang SA, Tam W, Tsai AG, Arber DA, Hasserjian RP, Geyer JT, et al. Targeted next-generation sequencing identifies a subset of idiopathic hypereosinophilic syndrome with features similar to chronic eosinophilic leukemia, not otherwise specified. Mod Pathol. 2016;29(8):854–64. This study showed that a significant proportion of patients diagnosed with “idiopathic” HES in fact have mutations in various housekeeping and signaling genes that are known to be associated with cancers.
Pardanani A, Lasho T, Wassie E, Finke C, Zblewski D, Hanson CA, et al. Predictors of survival in WHO-defined hypereosinophilic syndrome and idiopathic hypereosinophilia and the role of next-generation sequencing. Leukemia. 2016;30(9):1924–6.
Harfi I, Schandene L, Dremier S, Roufosse F. Eosinophils affect functions of in vitro-activated human CD3-CD4+ T cells. J Transl Med. 2013;11:112.
Jin JJ, Butterfield JH, Weiler CR. Hematologic malignancies identified in patients with hypereosinophilia and hypereosinophilic syndromes. J Allergy Clin Immunol Pract. 2015;3(6):920–5.
Andersen CL, Siersma VD, Hasselbalch HC, Lindegaard H, Vestergaard H, Felding P, et al. Eosinophilia in routine blood samples and the subsequent risk of hematological malignancies and death. Am J Hematol. 2013;88(10):843–7.
Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319–25.
Klion AD. Eosinophilia: a pragmatic approach to diagnosis and treatment. Hematology Am Soc Hematol Educ Program. 2015;2015:92–7.
Katre RS, Sunnapwar A, Restrepo CS, Katabathina VS, Mumbower A, Baxi A, et al. Cardiopulmonary and gastrointestinal manifestations of eosinophil- associated diseases and idiopathic hypereosinophilic syndromes: multimodality imaging approach. Radiographics. 2016;36(2):433–51.
Butterfield JH, Kane GC, Weiler CR. Hypereosinophilic syndrome: endomyocardial biopsy versus echocardiography to diagnose cardiac involvement. Postgrad Med. 2017;129(5):517–23.
Klion AD. How I treat hypereosinophilic syndromes. Blood. 2015;126(9):1069–77.
• Khoury P, Abiodun AO, Holland-Thomas N, Fay MP, Klion AD. Hypereosinophilic syndrome subtype predicts responsiveness to glucocorticoids. J Allergy Clin Immunol Pract. 2018;6(1):190–5. This study demonstrated that the myeloid and lymphocytic variants of HES are the least-responsive to treatment with glucocorticoids.
Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358(12):1215–28.
Roufosse FE, Kahn JE, Gleich GJ, Schwartz LB, Singh AD, Rosenwasser LJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. 2013;131(2):461–7.
Roufosse F, de Lavareille A, Schandene L, Cogan E, Georgelas A, Wagner L, et al. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126(4):828–35.
Schwarz C, Muller T, Lau S, Parasher K, Staab D, Wahn U. Mepolizumab—a novel option for the treatment of hypereosinophilic syndrome in childhood. Pediatr Allergy Immunol. 2018;29(1):28–33.
Guo C, Bochner BS. Mepolizumab as a successful steroid-sparing agent in two patients with idiopathic hypereosinophilic syndrome (iHES). J Allergy Clin Immunol. 2018;141(2):AB27.
Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G 3rd, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):456–63. 63
Murphy K, Jacobs J, Bjermer L, Fahrenholz JM, Shalit Y, Garin M, et al. Long-term safety and effficacy of reslizumab in patients with eosinophilic asthma. J Allergy Clin Immunol Pract. 2017;5(6):1572–81.
Klion AD, Law MA, Noel P, Kim YJ, Haverty TP, Nutman TB. Safety and efficacy of the monoclonal anti-interleukin-5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood. 2004;103(8):2939–41.
Teva announces top-line results from phase III studies of subcutaneously administered reslizumab in patients with severe eosinophilic asthma. http://www.tevapharm.com/news/teva_announces_top_line_results_from_phase_iii_studies_of_subcutaneously_administered_reslizumab_in_patients_with_severe_eosinophilic_asthma_01_18.aspx.
Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–53.
Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–27.
FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–41.
• Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–58. This is the most recently published phase 3 trial for benralizumab, confirming findings from the previous trials demonstrating benralizumab’s excellent eosinophil-depleting effects in severe eosinophilic asthma.
Kuang FL, Alao H, Kumar S, Powers A, Quezado M, Wang Z, et al. Benralizumab (anti-IL5Rα) depletes gut tissue eosinophilia and improves symptoms in hypereosinophilic syndrome with gastrointestinal involvement. J Allergy Clin Immunol. 2018;141((2):AB196.
Massanari M, Holgate ST, Busse WW, Jimenez P, Kianifard F, Zeldin R. Effect of omalizumab on peripheral blood eosinophilia in allergic asthma. Respir Med. 2010;104(2):188–96.
Busse W, Spector S, Rosen K, Wang Y, Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013;132(2):485–6.
Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–11.
Foroughi S, Foster B, Kim N, Bernardino LB, Scott LM, Hamilton RG, et al. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol. 2007;120(3):594–601.
Loizou D, Enav B, Komlodi-Pasztor E, Hider P, Kim-Chang J, Noonan L, et al. A pilot study of omalizumab in eosinophilic esophagitis. PLoS One. 2015;10(3):e0113483.
Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147(3):602–9.
Weinstein SF, Katial R, Jayawardena S, Pirozzi G, Staudinger H, Eckert L, et al. Efficacy and safety of dupilumab in perennial allergic rhinitis and comorbid asthma. J Allergy Clin Immunol. 2018 (in press).
Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44.
Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M, et al. Dupilumab efficacy and safety in adult patients with active eosinophilic esophagitis: a randomized double-blind placebo-controlled phase 2 trial. United Eur Gastro J. 2017;5(Suppl 1).
Pitini V, Teti D, Arrigo C, Righi M. Alemtuzumab therapy for refractory idiopathic hypereosinophilic syndrome with abnormal T cells: a case report. Br J Haematol. 2004;127(5):477.
Wagner LA, Speckart S, Cutter B, Gleich GJ. Treatment of FIP1L1/PDGFRA-negative hypereosinophilic syndrome with alemtuzumab, an anti-CD52 antibody. J Allergy Clin Immunol. 2009;123(6):1407–8.
Verstovsek S, Tefferi A, Kantarjian H, Manshouri T, Luthra R, Pardanani A, et al. Alemtuzumab therapy for hypereosinophilic syndrome and chronic eosinophilic leukemia. Clin Cancer Res. 2009;15(1):368–73.
Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–46.
Gleich GJ, Leiferman KM, Pardanani A, Tefferi A, Butterfield JH. Treatment of hypereosinophilic syndrome with imatinib mesylate. Lancet. 2002;359(9317):1577–8.
Ault P, Cortes J, Koller C, Kaled ES, Kantarjian H. Response of idiopathic hypereosinophilic syndrome to treatment with imatinib mesylate. Leuk Res. 2002;26(9):881–4.
Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348(13):1201–14.
• Gotlib J. Tyrosine kinase inhibitors in the treatment of eosinophilic neoplasms and systemic mastocytosis. Hematol Oncol Clin North Am. 2017;31(4):643–61. An excellent and thorough review of the field.
Helbig G. Imatinib for the treatment of hypereosinophilic syndromes. Expert Rev Clin Immunol. 2018;14(2):163–70.
Jovanovic JV, Score J, Waghorn K, Cilloni D, Gottardi E, Metzgeroth G, et al. Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood. 2007;109(11):4635–40.
Klion AD, Robyn J, Akin C, Noel P, Brown M, Law M, et al. Molecular remission and reversal of myelofibrosis in response to imatinib mesylate treatment in patients with the myeloproliferative variant of hypereosinophilic syndrome. Blood. 2004;103(2):473–8.
Helbig G, Moskwa A, Hus M, Piszcz J, Swiderska A, Urbanowicz A, et al. Durable remission after treatment with very low doses of imatinib for FIP1L1-PDGFRalpha-positive chronic eosinophilic leukaemia. Cancer Chemother Pharmacol. 2011;67(4):967–9.
Klion AD, Robyn J, Maric I, Fu W, Schmid L, Lemery S, et al. Relapse following discontinuation of imatinib mesylate therapy for FIP1L1/PDGFRA-positive chronic eosinophilic leukemia: implications for optimal dosing. Blood. 2007;110(10):3552–6.
Helbig G, Kyrcz-Krzemien S. Cessation of imatinib mesylate may lead to sustained hematologic and molecular remission in FIP1L1-PDGFRA-mutated hypereosinophilic syndrome. Am J Hematol. 2014;89(1):115.
Legrand F, Renneville A, Macintyre E, Mastrilli S, Ackermann F, Cayuela JM, et al. The spectrum of FIP1L1-PDGFRA-associated chronic eosinophilic leukemia: new insights based on a survey of 44 cases. Medicine (Baltimore). 2013;92(5):e1–e9.
Helbig G. Imatinib mesylate for unmutated hypereosinophilic syndromes: does it work? Eur J Intern Med. 2016;32:19–20.
Khoury P, Desmond R, Pabon A, Holland-Thomas N, Ware JM, Arthur DC, et al. Clinical features predict responsiveness to imatinib in platelet-derived growth factor receptor-alpha-negative hypereosinophilic syndrome. Allergy. 2016;71(6):803–10.
Lierman E, Cools J. Recent breakthroughs in the understanding and management of chronic eosinophilic leukemia. Expert Rev Anticancer Ther. 2009;9(9):1295–304.
Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8(2):15.
Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018 (in press);15:234–48.
Schwaab J, Knut M, Haferlach C, Metzgeroth G, Horny HP, Chase A, et al. Limited duration of complete remission on ruxolitinib in myeloid neoplasms with PCM1-JAK2 and BCR-JAK2 fusion genes. Ann Hematol. 2015;94(2):233–8.
Rumi E, Milosevic JD, Casetti I, Dambruoso I, Pietra D, Boveri E, et al. Efficacy of ruxolitinib in chronic eosinophilic leukemia associated with a PCM1-JAK2 fusion gene. J Clin Oncol. 2013;31(17):269–71.
Rumi E, Milosevic JD, Selleslag D, Casetti I, Lierman E, Pietra D, et al. Efficacy of ruxolitinib in myeloid neoplasms with PCM1-JAK2 fusion gene. Ann Hematol. 2015;94(11):1927–8.
• King B, Lee AI, Choi J. Treatment of hypereosinophilic syndrome with cutaneous involvement with the JAK inhibitors tofacitinib and ruxolitinib. J Invest Dermatol. 2017;137(4):951–4. This study demonstrated that JAK inhibitors can be effective for the treatment of certain forms of HES.
Roufosse F. Management of hypereosinophilic syndromes. Immunol Allergy Clin N Am. 2015;35(3):561–75.
Gotlib J. World Health Organization-defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am J Hematol. 2017;92(11):1243–59.
Cudkowicz M, Bozik ME, Ingersoll EW, Miller R, Mitsumoto H, Shefner J, et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med. 2011;17(12):1652–6.
• Dworetzky SI, Hebrank GT, Archibald DG, Reynolds IJ, Farwell W, Bozik ME. The targeted eosinophil-lowering effects of dexpramipexole in clinical studies. Blood Cells Mol Dis. 2017;63:62–5. This study makes the point that an oral agent, dexpramipexole, has a unique and remarkable ability to reduce eosinophil numbers.
Prussin C, Laidlaw TM, Panettieri RA, Ferguson BJ, Adappa ND, Lane AP, et al. Dexpramipexole effectively lowers blood and tissue eosinophils in subjects with chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology. 2017;139(Suppl Feb):AB64.
Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101(12):5014–20.
Carroll DJ, O'Sullivan JA, Nix DB, Cao Y, Tiemeyer M, Bochner BS. Siglec-8 is an activating receptor mediating beta2 integrin-dependent function in human eosinophils. J Allergy Clin Immunol 2018 (in press).
Youngblood BA, Brock EC, Leung J, Bebbington C, Tomasevic N. Treatment with an anti-Siglec-8 antibody reduces eosinophilic gastrointestinal inflammation in mice. Gordon Research Conference on Food Allergy. 2018; Ventura, CA.
Rasmussen HS, Chang AT, Tomasevic N, Bebbington C. Phase 1 double-blind, placebo-controlled, ascending dose study of Siglec-8 selective mAb AK002 in healthy subjects. J Allergy Clin Immunol. 2018;141(2):AB403.
Hamann J, Koning N, Pouwels W, Ulfman LH, van Eijk M, Stacey M, et al. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol. 2007;37(10):2797–802.
Legrand F, Tomasevic N, Simakova O, Lee CC, Wang Z, Raffeld M, et al. The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J Allergy Clin Immunol. 2014;133(5):1439–47.
Gonem S, Berair R, Singapuri A, Hartley R, Laurencin MFM, Bacher G, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699–707.
Straumann A, Hoesli S, Bussmann C, Stuck M, Perkins M, Collins LP, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68(3):375–85.
Pettipher R, Hunter MG, Perkins CM, Collins LP, Lewis T, Baillet M, et al. Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459. Allergy. 2014;69(9):1223–32.
Korenblat P, Kerwin E, Leshchenko I, Yen K, Holweg CTJ, Anzures-Cabrera J, et al. Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respir Med. 2018;134:143–9.
Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365(12):1088–98.
Molfino NA, Kuna P, Leff JA, Oh CK, Singh D, Chernow M, et al. Phase 2, randomised placebo-controlled trial to evaluate the efficacy and safety of an anti-GM-CSF antibody (KB003) in patients with inadequately controlled asthma. BMJ Open. 2016;6(1):007709.
Neighbour H, Boulet LP, Lemiere C, Sehmi R, Leigh R, Sousa AR, et al. Safety and efficacy of an oral CCR3 antagonist in patients with asthma and eosinophilic bronchitis: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2014;44(4):508–16.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Bochner has current or recent consulting or scientific advisory board arrangements with or has received honoraria from Sanofi-Aventis, TEVA, GlaxoSmithKline, AstraZeneca, and Allakos, and owns stock in Allakos. He receives publication-related royalty payments from Elsevier and UpToDate™ and is a co-inventor on existing Siglec-8-related patents and thus may be entitled to a share of royalties received by Johns Hopkins University on the potential sales of such products. Dr. Bochner is also a co-founder of Allakos, which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies. Melanie C. Dispenza declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Myeloproliferative Neoplasms
Rights and permissions
About this article
Cite this article
Dispenza, M.C., Bochner, B.S. Diagnosis and Novel Approaches to the Treatment of Hypereosinophilic Syndromes. Curr Hematol Malig Rep 13, 191–201 (2018). https://doi.org/10.1007/s11899-018-0448-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-018-0448-8