Abstract
New targeted therapies administered in phase II and phase III studies have produced substantial improvements in outcomes of myelofibrosis (MF). However, strong documentation that the new agents modify the natural history of the disease is lacking, and a number of therapeutic indications of new drugs remain unaddressed. Overall survival (OS) improvement is the major goal of next-generation clinical trials in MF. This may be attained if an adequate population of patients and an unambiguous design of the trial will be selected. Another goal is preventing disease progression in early MF: this requires a controlled clinical trial with an accessible endpoint and a clinically relevant definition of disease progression. Improvement in the documentation of responsiveness of patient-reported outcomes (PROs) will allow to use them as a critical endpoint of new trials. Finally, exploiting the clinical utility of biomarkers should become a major goal of future clinical experimentation in MF.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Swerdlow SH, Campo E, Harris NL, et al (eds). WHO classification of tumours of haemopoietic and lymphoid tissues. IARC: Lyon 2008.
Tefferi A. Primary myelofibrosis: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88:141–50.
Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761–70.
Vainchenker W, Delhommeau F, Constantinescu SN, et al. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;18:1723–35.
Tenedini E, Bernardis I, Artusi V, et al. Targeted cancer exome sequencing reveals recurrent mutations in myeloproliferative neoplasms. Leukemia. 2014;28:1052–10159.
Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–405.
Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–90.
Quintás-Cardama A, Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clin Cancer Res. 2013;19:1933–40.
Guglielmelli P, Barosi G, Rambaldi A, et al. Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood. 2011;118:2069–76.
Bogani C, Bartalucci N, Martinelli S, et al. mTOR inhibitors alone and in combination with JAK2 inhibitors effectively inhibit cells of myeloproliferative neoplasms. PLoS One. 2013;8:e54826.
Fiskus W, Verstovsek S, Manshouri T, et al. Dual PI3K/AKT/mTOR inhibitor BEZ235 synergistically enhances the activity of JAK2 inhibitor against cultured and primary human myeloproliferative neoplasm cells. Mol Cancer Ther. 2013;12:577–88.
Tabarroki A, Tiu RV. Immunomodulatory agents in myelofibrosis. Expert Opin Investig Drugs. 2012;21:1141–54.
Pang M, Zhuang S. Histone deacetylase: a potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther. 2010;335:266–72.
Quintás-Cardama A, Kantarjian H, Estrov Z, et al. Therapy with the histone deacetylase inhibitor pracinostat for patients with myelofibrosis. Leuk Res. 2012;36:1124–7.
Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood. 2010;116:3735–42.
Tefferi A, LaPlant BR, Begna K, et al. Imetelstat, a telomerase inhibitor, therapy for myelofibrosis: a pilot study. Blood. 2014; 124(21) abstract n. 653.
Barosi G, Tefferi A, Besses C, et al. Clinical end points for drug treatment trials in BCR-ABL1-negative classic myeloproliferative neoplasms: consensus statements from European LeukemiaNET (ELN) and Internation Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT). Leukemia. 2015;29:20–6.
Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) & European LeukemiaNet (ELN) consensus report. Blood. 2013;122:1395–8.
Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098–103.
Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–901.
Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115:1703–8.
Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–7.
Vannuccchi AM, Guglielmelli P, Rotunno G, et al. Mutation-enhanced international prognostic scoring system (MIPSS) for primary myelofibrosis: an AGIMM & IWG-MRT project. Blood 2014;124, abstract n. 403.
Barosi G, Gattoni E, Guglielmelli P, et al. Phase I/II study of single-agent bortezomib for the treatment of patients with myelofibrosis. Clinical and biological effects of proteasome inhibition. Am J Hematol. 2010;85:616–9.
Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–27.
Apostolidou E, Kantarjian H, Thomas D, et al. Phase II study of sunitinib in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Clin Lymphoma Myeloma Leuk. 2010;10:281–4.
Parikh SA, Kantarjian H, Schimmer A, et al. Phase II study of obatoclax mesylate (GX15-070), a small-molecule BCL-2 family antagonist, for patients with myelofibrosis. Clin Lymphoma Myeloma Leuk. 2010;10:285–9.
Santos FP, Kantarjian HM, Jain N, et al. Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood. 2010;115:1131–6.
Rambaldi A, Dellacasa CM, Finazzi G, et al. A pilot study of the histone-deacetylase inhibitor givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol. 2010;150:446–55.
Pardanani A, Gotlib JR, Jamieson C, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29:789–96.
Mascarenhas J, Lu M, Li T, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF). Br J Haematol. 2013;161:68–75.
DeAngelo DJ, Mesa RA, Fiskus W, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol. 2013;162:326–35.
Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27:1322–7.
Mesa RA, Silver RT, Verstovsek S, et al. Single agent bevacizumab for myelofibrosis: results of the Myeloproliferative Disorders Research Consortium Trial. Haematologica. 2013;98:1421–3.
Talpaz M, Paquette R, Afrin L, et al. Interim analysis of safety and efficacy of ruxolitinib in patients with myelofibrosis and low platelet counts. J Hematol Oncol. 2013;6:81.
Verstovsek S, Tam CS, Wadleigh M, et al. Phase I evaluation of XL019, an oral, potent, and selective JAK2 inhibitor. Leuk Res. 2014;38:316–22.
Foran J, Ravandi F, Wierda W, et al. A phase I and pharmacodynamic study of AT9283, a small-molecule inhibitor of aurora kinases in patients with relapsed/refractory leukemia or myelofibrosis. Clin Lymphoma Myeloma Leuk. 2014;14:223–30.
Pardanani A, Tefferi A, Guglielmelli P, et al. Evaluation of plitidepsin in patients with primary myelofibrosis and post polycythemia vera/essential thrombocythemia myelofibrosis: results of preclinical studies and a phase II clinical trial. Blood Cancer J. 2015;5:e286.
Andersen CL, Mortensen NB, Klausen TW, et al. A phase II study of vorinostat (MK-0683) in patients with primary myelofibrosis and post-polycythemia vera myelofibrosis. Haematologica. 2014;99:e5–7.
Komrokji RS, Seymour JF, Roberts AW, Wadleigh M, To LB, Scherber R, et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood. 2015;125:2649–55.
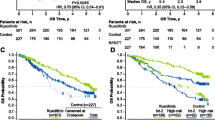
Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. This study led to FDA and EMA approval of ruxolitinib for patients with intermediate or high risk MF or with splenomegaly or symptoms.
Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98. This study led to FDA and EMA approval of ruxolitinib for patients with intermediate or high risk MF or with splenomegaly or symptoms.
Gupta V, Gotlib J, Radich JP, et al. Janus kinase inhibitors and allogeneic stem cell transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2014;20:1274–81.
Jaekel N, Behre G, Behning A, et al. Allogeneic hematopoietic cell transplantation for myelofibrosis in patients pretreated with the JAK1 and JAK2 inhibitor ruxolitinib. Bone Marrow Transplant. 2014;49:179–84.
Stübig T, Alchalby H, Ditschkowski M, et al. JAK inhibition with ruxolitinib as pretreatment for allogeneic stem cell transplantation in primary or post-ET/PV myelofibrosis. Leukemia. 2014;28:1736–8.
Alchalby H, Kröger N. Allogeneic stem cell transplant vs. Janus kinase inhibition in the treatment of primary myelofibrosis or myelofibrosis after essential thrombocythemia or polycythemia vera. Clin Lymphoma Myeloma Leuk. 2014;14 Suppl:S36-41.
Spoerl S, Mathew NR, Bscheider M, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123:3832–42.
Ballinger TJ, Savani BN, Gupta V, et al. How we manage JAK inhibition in allogeneic transplantation for myelofibrosis. Eur J Haematol. 2015;94:115–9.
Pieri L, Paoli C, Arena U, et al. A phase 2 study of ruxolitinib in patients with splanchnic vein thrombosis associated with myeloproliferative neoplasm: a study from the AGIMM group. Blood 2014 (21) Abstract n. 72125.
Barosi G, Rosti V, Gale RP. Critical appraisal of the role of ruxolitinib in myeloproliferative neoplasm-associated myelofibrosis. Oncotarget and Therapy, accepted for publication (2015).
Martí-Carvajal AJ, Anand V, Solà I. Janus kinase-1 and Janus kinase-2 inhibitors for treating myelofibrosis. Cochrane Database Syst Rev. 2015 Apr 10; [Epub ahead of print].
Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283–93.
Sobrero AF, Pastorino A, Sargent DJ, Bruzzi P. Raising the bar for antineoplastic agents: how to choose threshold values for superiority trials in advanced solid tumors. Clin Cancer Res. 2015;21:1036–43.
Ellis LM, Bernstein DS, Voest EE, et al. American Society of Clinical Oncology perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol. March 17, 2014 (early release online).
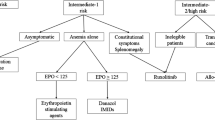
Hasselbalch HC. Myelofibrosis with myeloid metaplasia: the advanced phase of an untreated disseminated hematological cancer. Time to change our therapeutic attitude with early upfront treatment? Leuk Res. 2009;33:11–8.
Silver RT, Vandris K, Goldman JJ. Recombinant interferon-α may retard progression of early primary myelofibrosis: a preliminary report. Blood. 2011;117:6669–72.
Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90:1128–32.
Gupta V, Foltz LM, Sirhan S, Turner AR, Busque L. Response criteria for “progressive disease” for myelofibrosis: are the current criteria satisfactory? E-letter, Blood 11 September 2013.
Tam CS, Kantarjian H, Cortes J, et al. Dynamic model for predicting death within 12 months in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. J Clin Oncol. 2009;27:5587–93.
Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–9.
Harada-Shirado K, Ikeda K, Ogawa K, et al. Dysregulation of the MIRLET7/HMGA2 axis with methylation of the CDKN2A promoter in myeloproliferative neoplasms. Br JHaematol. 2015;168:338–49.
Guglielmelli P, Zini R, Bogani C, et al. Molecular profiling of CD34+ cells in idiopathic myelofibrosis identifies a set of disease-associated genes and reveals the clinical significance of Wilms’ tumor gene 1 (WT1). Stem Cells. 2007;25:165–73.
Benati M, Montagnana M, Danese E, et al. Role of JAK2 V617F mutation and aberrant expression of microRNA-143 in myeloproliferative neoplasms. Clin Chem Lab Med. 2014. [Epub ahead of print].
Norfo R, Zini R, Pennucci V, Bianchi E, Salati S, Guglielmelli P. miRNA-mRNA integrative analysis in primary myelofibrosis CD34+ cells: role of miR-155/JARID2 axis in abnormal megakaryopoiesis. Blood. 2014;124:e21–32.
Zhan H, Cardozo C, Raza A. MicroRNAs in myeloproliferative neoplasms. Br J Haematol. 2013;161:471–83.
Gebauer N, Bernard V, Gebauer W, Feller AC, Merz H. MicroRNA expression and JAK2 allele burden in bone marrow trephine biopsies of polycythemia vera, essential thrombocythemia and early primary myelofibrosis. Acta Haematol. 2013;129:251–6.
Albano F, Anelli L, Zagaria A, Coccaro N, Casieri P, Minervini A, et al. SETBP1 and miR_4319 dysregulation in primary myelofibrosis progression to acute myeloid leukemia. J Hematol Oncol. 2012;5:48.
Girardot M, Pecquet C, Boukour S, et al. miR-28 is a thrombopoietin receptor targeting microRNA detected in a fraction of myeloproliferative neoplasm patient platelets. Blood. 2010;116:437–45.
Hussein K, Theophile K, Dralle W, Wiese B, Kreipe H, Bock O. MicroRNA expression profiling of megakaryocytes in primary myelofibrosis and essential thrombocythemia. Platelets. 2009;20:391–400.
Guglielmelli P, Tozzi L, Pancrazzi A, et al. MicroRNA expression profile in granulocytes from primary myelofibrosis patients. Exp Hematol. 2007;35:1708–18.
Barosi G, Viarengo G, Pecci A, et al. Diagnostic and clinical relevance of the number of circulating CD34(+) cells in myelofibrosis with myeloid metaplasia. Blood. 2001;98:3249–55.
Passamonti F, Vanelli L, Malabarba L, et al. Clinical utility of the absolute number of circulating CD34-positive cells in patients with chronic myeloproliferative disorders. Haematologica. 2003;88:1123–9.
Passamonti F, Rumi E, Pietra D, et al. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107:3676–82.
Arora B, Sirhan S, Hoyer JD, Mesa RA, Tefferi A. Peripheral blood CD34 count in myelofibrosis with myeloid metaplasia: a prospective evaluation of prognostic value in 94 patients. Br J Haematol. 2005;128:42–8.
Barbui T, Carobbio A, Finazzi G, et al. Elevated C-reactive protein is associated with shortened leukemia-free survival in patients with myelofibrosis. Leukemia. 2013;27:2084–6.
Pardanani A, Lasho TL, Finke CM, et al. Polyclonal immunoglobulin free light chain levels predict survival in myeloid neoplasms. J Clin Oncol. 2012;30:1087–94.
Pardanani A, Finke C, Abdelrahman RA, Lasho TL, Tefferi A. Associations and prognostic interactions between circulating levels of hepcidin, ferritin and inflammatory cytokines in primary myelofibrosis. Am J Hematol. 2013;88:312–6.
Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29:1356–63.
Dalton WS, Friend SH. Cancer biomarkers—an invitation to the table. Science. 2006;312:1165–8.
Febbo PG, Ladanyi M, Aldape KD, et al. NCCN Task Force report: evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw. 2011;9 Suppl 5:S1–32.
Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Accelerating the development of biomarkers for drug safety: workshop summary. Washington (DC): National Academies Press (US); 2009.
Acknowledgments
GB was supported by a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC, Milano) “Special Program Molecular Clinical Oncology 5x1000” to AGIMM (AIRC-Gruppo Italiano Malattie Mieloproliferative).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Giovanni Barosi declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Myeloproliferative Disorders
Rights and permissions
About this article
Cite this article
Barosi, G. Setting Appropriate Goals for the Next Generation of Clinical Trials in Myelofibrosis. Curr Hematol Malig Rep 10, 362–369 (2015). https://doi.org/10.1007/s11899-015-0281-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-015-0281-2