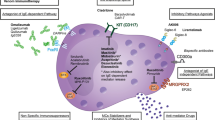
Abstract
World Health Organization-defined myeloproliferative neoplasms share a common pathobiologic theme of constitutive activation of tyrosine kinases (TKs). While myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRA or PDGFRB exhibit exquisite responsiveness to imatinib, other eosinophilic disorders such as chronic eosinophilic leukemia—not otherwise specified (CEL-NOS) and idiopathic hypereosinophilic syndrome (HES) lack recurrent gene mutations or known druggable targets. In systemic mastocytosis (SM), KIT D816V is identified in ∼90 % of patients, but demonstrates imatinib resistance. Recently, the multikinase/KIT inhibitor midostaurin (PKC412) has demonstrated encouraging activity in patients with advanced SM, and selective KIT D816V inhibitors are entering clinical development. Pre-clinical rationale also exists for use of small molecule inhibitors of TK-linked pathways (e.g., BTK, JAK-STAT, PI3K/AKT, and FGFR1) that are implicated in normal or dysregulated signaling in eosinophils or mast cells. A complementary therapeutic approach is the use of naked antibody (e.g., mepolizumab and alemtuzumab) or antibody-based drug immunoconjugates (brentuximab vedotin) against targets expressed on the surface of eosinophils or mastocytes that can block proliferation and/or induce apoptosis of these cells. Ultimately, biologic and molecular characterization of eosinophilia and SM cases will help to optimize selection of TK inhibitors or therapeutic antibodies for individual patients.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ackerman SJ, Butterfield JH. Eosinophilia, eosinophil-associated diseases, chronic eosinophilic leukemia, and the hypereosinophilic syndromes. In: Hoffman R, Benz Jr E, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P, editors. Hematology: Basic Principles and Practice. 4th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. p. 763–86.
Brito-Babapulle F. The eosinophilias, including the idiopathic hypereosinophilic syndrome. Br J Haematol. 2003;121(2):203–23.
Bain BJ, Gilliland DG, Horny H-P, Vardiman JW. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, or FGFR1. In: Swerdlow S, Harris NL, Stein H, Jaffe ES, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. p. 68–73.
Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. Am J Hematol. 2014;89(3):325–37.
Savage N, George TI, Gotlib J. Myeloid neoplasms associated with eosinophilia and rearrangement of PDGFRA, PDGFRB, and FGFR1: a review. Int J Lab Hematol. 2013;35(5):491–500.
Bain BJ, Gilliland DG, Horny H-P, Vardiman JW. Chronic eosinophilic leukaemia, not otherwise specified. In: Swerdlow S, Harris NL, Stein H, Jaffe ES, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. p. 51–3.
Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319–25.
Horny HP, Metcalfe DD, Bennet JM, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. 4th ed. IARC: Lyon; 2008. p. 54–63.
Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727–36.
Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121(8):1285–95. Largest retrospective cohort of patients with the mast cell leukemia.
Valent P, Sotlar K, Sperr WR, Escribano L, Yavuz S, Reiter A, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014;25(9):1691–700.
Sperr WR, Horny HP, Valent P. Spectrum of associated clonal hematologic non-mast cell lineage disorders occurring in patients with systemic mastocytosis. Int Arch Allergy Immunol. 2002;127(2):140–2.
Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90(3):250–62.
Pardanani A. How I, treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage). Blood. 2013;121(16):3085–94.
Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32(29):3264–74. This multicenter, retrospective analysis of 57 patients is the first aggregate report of the allogeneic stem cell transplant experience in this rare patient population.
Arock M, Sotlar K, Broesby-Olsen S, Hoermann G, Escribano L, Kristensen TK, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015;29:1223–32.
Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103(8):3222–5.
Álvarez-Twose I, González P, Morgado JM, Jara-Acevedo M, Sánchez-Muñoz L, Matito A, et al. Complete response after imatinib mesylate therapy in a patient with well-differentiated systemic mastocytosis. J Clin Oncol. 2012;30(12):e126–9.
Zhang LY, Smith ML, Schultheis B, Fitzgibbon J, Lister TA, Melo JV, et al. A novel K509I mutation of KIT identified in familial mastocytosis – in vitro and in vivo responsiveness to imatinib therapy. Leuk Res. 2006;30(4):373–8.
Hoffmann KM, Moser A, Lohse P, Winkler A, Binder B, Sovinz P, et al. Successful treatment of progressive cutaneous mastocytosis with imatinib in a 2-year-old boy carrying a somatic KIT mutation. Blood. 2008;112(5):1655–7.
Mital A, Piskorz A, Lewandowski K, Wasąg B, Limon J, Hellmann A. A case of mast cell leukemia with exon 9 KIT mutation and good response to imatinib. Eur J Haematol. 2011;86(6):531–5.
Verstovsek S, Tefferi A, Cortes J, O’Brien S, Garcia-Manero G, Pardanani A, et al. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clin Cancer Res. 2008;14(12):3906–15.
Gotlib J, Berubé C, Growney JD, Chen CC, George TI, Williams C, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106(8):2865–70.
Gotlib J, DeAngelo DJ, George TI, et al. KIT inhibitor midostaurin exhibits a high rate of clinically meaningful and durable responses in advanced systemic mastocytosis: report of a fully accrued phase II trial. Blood (ASH Annual Meeting Abstracts). 2010;116:316.
Gotlib J, Kluin-Nelemans HC, George TI, et al. Midostaurin (PKC412) Demonstrates a High Rate of Durable Responses in Patients with Advanced Systemic Mastocytosis: Results from the Fully Accrued Global Phase 2 CPKC412D2201 Trial. Blood (ASH Annual Meeting Abstracts). 2014;124:636. Report of the multikinase/KIT inhibitor midostaurin in advanced SM details high rates of reversion of organ damage, reduction of bone marrow mast cell burden and serum tryptase levels, and improvement of symptoms/quality of life.
Krauth MT, Mirkina I, Herrmann H, Baumgartner C, Kneidinger M, Valent P. Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells. Clin Exp Allergy. 2009;39(11):1711–20.
Evans EK, Hodous BL, Gardino A, et al. First Selective KIT D816V Inhibitor for Patients with Systemic Mastocytosis. Blood (ASH Annual Meeting Abstracts). 2014;124:3217.
Felices M, Falk M, Kosaka Y, Berg LJ. Tec kinases in T cell and mast cell signaling. Adv Immunol. 2007;93:145–84.
Iwaki S, Tkaczyk C, Satterhwaite AB, Halcomb K, Beaven MA, Metcalfe DD, et al. Btk plays a crucial role in the amplification of Fc epsilonR1-mediated mast cell activation by Kit. J Biol Chem. 2005;280(48):40261–70.
Hata D, Kawakami Y, Inagaki N, Lantz CS, Kitamura T, Khan WN, et al. Involvement of Bruton's tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187(8):1235–47.
Kuehn HS, Rådinger M, Brown JM, Ali K, Vanhaesebroeck B, Beaven MA, et al. Btk-dependent Rac activation and actin rearrangement following FcεRI aggregation promotes enhanced chemotactic responses of mast cells. J Cell Sci. 2010;123(15):2576–85.
Gleixner KV, Mayerhofer M, Cerny-Reiterer S, Hörmann G, Rix U, Bennett KL, et al. KIT-D816V-independent oncogenic signaling in neoplastic cells in systemic mastocytosis: role of Lyn and Btk activation and disruption by dasatinib and bosuntinib. Blood. 2011;118(7):1885–98. Characterization of aberrant BTK (and Lyn) signaling independent of KIT D816V in malignant mast cells, and demonstration that siRNAs and tyrosine kinase inhibitors against BTK can decrease mast cell growth and survival.
Soucek L, Buggy JJ, Kortlever R, Adimoolam S, Monclús HA, Allende MTS, et al. Modeling pharmacologic inhibition of mast cell degranulation as a therapy for insulinoma. Neoplasia. 2011;13(11):1093–100.
Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106(6):2162–8.
Helbig G, Stella-Holowiecka B, Majewski M, Lewandowska M, Holowiecki J. Interferon ɑ induces a good molecular response in a patient with chronic eosinophilic leukemia (CEL) carrying the JAK2V617F point mutation. Haematologica. 2007;92(11):e118–9.
Simon HU, Yousefi S, Dibbert B, Levi-Schaffer F, Blaser K. Anti-apoptotic signals of granulocyte-macrophage colony-stimulating factor are transduced via Jak2 tyrosine kinase in eosinophils. Eur J Immunol. 1997;27(12):3536–9.
Miike S, Nakao A, Hiraguri M, Kurasawa K, Saito Y, Iwamoto I. Involvement of JAK2, but not PI3-kinase/Akt and MAP kinase pathways, in anti-apoptotic signals of GM-CSF of human eosinophils. J Leukoc Biol. 1999;65(5):700–6.
Li B, Zhang G, Li C, He D, Li X, Zhang C, et al. Identification of JAK2 as a mediator of FIP1L1-PDGFRA-induced eosinophil growth and function in CEL. PLoS One. 2012;7(4):e34912.
Sotlar K, Bache A, Stellmacher F, Bültmann B, Valent P, Horny HP. Systemic mastocytosis associated with chronic idiopathic myelofibrosis: a distinct subtype of systemic mastocytosis associated with a clonal hematological non-mast cell lineage disorder carrying the activating point mutations KITD816V and JAK2V617F. J Mol Diagn. 2008;10(1):58–66.
Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122(14):2460–6. Advanced SM patients, especially patients with SM-AHNMD, often exhibit multiple myeloid gene mutations in addition to KIT D816V, which is associated with worse survival. JAK2 V617F is identified in selected cases.
Linnekin D, Weiler SR, Mou S, DeBerry CS, Keller JR, Ruscetti FW, et al. JAK2 is constitutively associated with c-Kit and is phosphorylated in response to stem cell factor. Acta Haematol. 1996;95(3-4):224–8.
Weiler SR, Mou S, DeBerry CS, Keller JR, Ruscetti FW, Ferris DK, et al. JAK2 is associated with the c-kit proto-oncogene product and is phosphorylated in response to stem cell factor. Blood. 1996;87(9):3688–93.
Brizzi MF, Zini MG, Aronica MG, Blechman JM, Yarden Y, Pegoraro L. Convergence of signaling by interleukin-3, granulocyte-macrophage colony-stimulating factor, and mast cell growth factor on JAK2 tyrosine kinase. J Biol Chem. 1994;269(50):31680–4.
Tagaya Y, Burton JD, Miyamoto Y, Waldmann TA. Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO J. 1996;15(18):4928–39.
Radosevic N, Winterstein D, Keller JR, Neubauer H, Pfeffer K, Linnekin D. JAK2 contributes to the intrinsic capacity of primary hematopoietic cells to respond to stem cell factor. Exp Hematol. 2004;32(2):149–56.
Sur R, Hall J, Cavender D, Malaviya R. Role of Janus kinase-2 in IgE receptor-mediated leukotriene C4 production by mast cells. Biochem Biophys Res Commun. 2009;390(3):786–90.
Lasho T, Tefferi A, Pardanani A. Inhibition of JAK-STAT signaling by TG101348: a novel mechanism for inhibition of KITD816V-dependent growth in mast cell leukemia cells. Leukemia. 2010;24(7):1378–80.
Harir N, Boudot C, Friedbichler K, Sonneck K, Kondo R, Martin-Lannerée S, et al. Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade. Blood. 2008;112(6):2463–73.
Chian R, Young S, Danilkovitch-Miagkova A, Rönnstrand L, Leonard E, Ferrao P, et al. Phosphatidylinositol 3 kinase contributes to the transformation of hematopoietic cells by the D816V c-Kit mutant. Blood. 2001;98(5):1365–73.
Kim MS, Rådinger M, Gilfillan AM. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008;29(10):493–501.
Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, et al. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431(7011):1007–11.
Ali K, Camps M, Pearce WP, Ji H, Rückle T, Kuehn N, et al. Isoform-specific functions of phosphoinositide 3-kinases: p110δ but not p110γ promotes optimal allergic responses in vivo. J Immunol. 2008;180(4):2538–44.
Koyasu S, Minowa A, Terauchi Y, Kadowaki T, Matsuda S. The role of phosphoinositide-3-kinase in mast cell homing to the gastrointestinal tract. In Novartis Foundation Symp. 2005;271:152–61.
Shivakrupa R, Bernstein A, Watring N, Linnekin D. Phosphatidylinositol 3'-kinase is required for growth of mast cells expressing the kit catalytic domain mutant. Cancer Res. 2003;63(15):4412–9.
Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. The phospholipase Cγ 1-dependent pathway of FcεRI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J Biol Chem. 2003;278(48):48474–84.
Marquardt DL, Alongi JL, Walker LL. The phosphatidylinositol 3-kinase inhibitor wortmannin blocks mast cell exocytosis but not IL-6 production. J Immunol. 1996;156(5):1942–5.
Kim MS, Kuehn HS, Metcalfe DD, Gilfillan AM. Activation and function of the mTORC1 pathway in mast cells. J Immunol. 2008;180(7):4586–95.
Blatt K, Herrmann H, Mirkina I, Hadzijusufovic E, Peter B, Strommer S, et al. The PI3-kinase/mTOR-targeting drug NVP-BEZ235 inhibits growth and IgE-dependent activation of human mast cells and basophils. PLoS One. 2012;7(1), e29925.
Chase A, Bryant C, Score J, Cross NC. Ponatinib as targeted therapy for FGFR1 fusions associated with the 8p11 myeloproliferative syndrome. Haematologica. 2013;98(1):103–6.
Ren M, Qin H, Ren R, Cowell JK. Ponatinib suppresses the development of myeloid and lymphoid malignancies associated with FGFR1 abnormalities. Leukemia. 2013;27(1):32–40.
Chase A, Grand FH, Cross NC. Activity of TKI258 against primary cells and cell lines with FGFR1 fusion genes associated with the 8p11 myeloproliferative syndrome. Blood. 2007;110(10):3729–34.
Wakim JJ, Tirado CA, Chen W, Collins R. t(8;22)/BCR-FGFR1 myeloproliferative disorder presenting as B-acute lymphoblastic leukemia: report of a case treated with sorafenib and review of the literature. Leuk Res. 2011;35(9):e151–3.
Chen J, DeAngelo DJ, Kutok JL, Williams IR, Lee BH, Wadleigh M, et al. PKC412 inhibits the zinc finger 198-fibroblast growth factor receptor 1 fusion tyrosine kinase and is active in treatment of stem cell myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004;101(40):14479–84.
Khodadoust M, Luo B, Medeiros C, et al. Clinical activity of ponatinib in a patient with FGFR1-rearranged mixed phenotype acute leukemia. Leukemia. 2015. doi:10.1038/leu.2015.136. First demonstration in a patient with a FGFR1-rearranged neoplasm of the activity of ponatinib, an FGFR1 inhibitor.
Hart TK, Cook RM, Zia-Amirhosseini P, Minthorn E, Sellers TS, Maleeff BE, et al. Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J Allergy Clin Immunol. 2001;108(2):250–7.
Plötz SG, Simon HU, Darsow U, Simon D, Vassina E, Yousefi S, et al. Use of an anti-interleukin-5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med. 2003;349(24):2334–9.
Garrett JK, Jameson SC, Thomson B, Collins MH, Wagoner LE, Freese DK, et al. Anti-interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol. 2004;113(1):115–9.
Klion AD, Law MA, Noel P, Kim YJ, Haverty TP, Nutman TB. Safety and efficacy of the monoclonal anti-interleukin-5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood. 2004;103(8):2939–41.
Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. New Engl J Med. 2008;358(12):1215–28.
Roufosse FE, Kahn JE, Gleich GJ, Schwartz LB, Singh AD, Rosenwasser LJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. 2013;131(2):461–7.
Pitini V, Teti D, Arrigo C, Righi M. Alemtuzumab therapy for refractory idiopathic hypereosinophilic syndrome with abnormal T-cells: a case report. Br J Haematol. 2004;127(5):477.
Sefcick A, Sowter D, DasGupta E, Russel NH, Byrne JL. Alemtuzumab therapy for refractory idiopathic hypereosinophilic syndrome. Br J Haematol. 2004;124(4):558–9.
Verstovsek S, Tefferi A, Kantarjian H, Manshouri T, Luthra R, Pardanani A, et al. Alemtuzumab therapy for hypereosinophilic syndrome and chronic eosinophilic leukemia. Clin Cancer Res. 2009;15(1):368–73.
Strati P, Cortes J, Faderl S, Kantarjian H, Verstovsek S. Long-term follow-up of patients with hypereosinophilic syndrome treated with alemtuzumab, an anti-CD52 antibody. Clin Lymphoma Myeloma Leuk. 2013;13(3):287–91. Longitudinal follow-up of patients with HES demonstrating that maintenance therapy with alemtuzumab is important for durable responses.
Santos DD, Hatjiharissi E, Tournilhac O, Chemaly MZ, Leleu X, Xu L, et al. CD52 is expressed on human mast cells and is a potential therapeutic target in Waldenstrom's Macroglobulinemia and mast cell disorders. Clin Lymphoma Myeloma. 2006;6(6):478–83.
Hoermann G, Blatt K, Greiner G, Putz EM, Berger A, Herrmann H, et al. CD52 is a molecular target in advanced systemic mastocytosis. FASEB J. 2014;28(8):3540–51.
Deutsch YE, Tadmor T, Podack ER, Rosenblatt JD. CD30: an important new target in hematologic malignancies. Leuk Lymphoma. 2011;52(9):1641–54.
Wahl AF, Klussman K, Thompson JD, Chen JH, Francisco LV, Risdon G, et al. The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin's disease. Cancer Res. 2002;62(13):3736–42.
Sotlar K, Cerny-Reiterer S, Petat-Dutter K, Hessel H, Berezowska S, Müllauer L, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24(4):585–95. First report highlighting the aberrant expression of CD30 on neoplastic mast cells.
Valent P, Sotlar K, Horny HP. Aberrant expression of CD30 in aggressive systemic mastocytosis and mast cell leukemia: a differential diagnosis to consider in aggressive hematopoietic CD30-positive neoplasms. Leuk Lymphoma. 2011;52(5):740–4.
Morgado JM, Perbellini O, Johnson RC, Teodósio C, Matito A, Álvarez-Twose I, et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology. 2013;63(6):780–7.
van Anrooij B, Kluin PM, Elberlink JNO, Kluin-Nelemans JC. CD30 in systemic mastocytosis. Immunol Allergy Clin N Am. 2014;34(2):341–55.
Katz J, Janik JE, Younes A. Brentuximab Vedotin (SGN-35). Clin Cancer Res. 2011;17(20):6428–36.
Gualberto A. Brentuximab Vedotin (SGN-35), an antibody-drug conjugate for the treatment of CD30-positive malignancies. Expert Opin Investig Drugs. 2012;21(2):205–16.
Pardanani A, Lasho T, Chen D, Limlinger TK, Finke C, Zblewski D, et al. Aberrant expression of CD123 (interleukin-3 receptor-α) on neoplastic mast cells. Leukemia. 2015;29(7):1605-8. doi:10.1038/leu.2015.16. Pre-clinical characterization of abnormally increased expression of CD123 on malignant mast cells which forms the basis of evaluating anti-CD123 antibody therapy for SM.
Frankel AE, Wooh JH, Ahn C, Pemmaraju N, Medeiros BC, Carraway HE, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood. 2014;124(3):385–92.
Krauth MT, Bohm A, Agis H, et al. Effects of the CD33-targeted drug gemtuzumab ozogamicin (Mylotarg) on growth and mediator secretion in human mast cells and blood basophils. Exp Hematol. 2007;35(1):108–16.
Karra L, Berent-Maoz B, Ben-Zimra M, Levi-Schaffer F. Are we ready to downregulate mast cells? Curr Opin Immunol. 2009;21(6):708–14.
Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12(4):345–55.
Berent-Maoz B, Piliponsky AM, Daigle I, Simon HU, Levi-Schaffer F. Human mast cells undergo TRAIL-induced poptosis. J Immunol. 2006;176(4):2272–8.
Berent-Maoz B, Salemi S, Mankuta D, Simon HU, Levi-Schaffer F. TRAIL mediated signaling in human mast cells: the influence of IgE-dependent activation. Allergy. 2008;63(3):333–40.
Malbec O, Daëron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol Rev. 2007;217(1):206–21.
Katz HR. Inhibition of pathologic inflammation by leukocyte Ig- like receptor B4 and related inhibitory receptors. Immunol Rev. 2007;217(1):222–30.
Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J Immunol. 2005;175(12):7989–95.
Bachelet I, Munitz A, Berent-Maoz B, Mankuta D, Levi-Schaffer F. Suppression of normal and malignant kit signaling by a bispecific antibody linking kit with CD300a. J Immunol. 2008;180(9):6064–9.
Munitz A, Bachelet I, Eliashar R, Moretta A, Moretta L, Levi- Schaffer F. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood. 2006;107(5):1996–2003.
Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8 A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275(2):861–6.
Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D’Alessio KJ, et al. Identification of SAF-2, a novel Siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 2000;105(6):1093–100.
Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39(3):317–24.
Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101(12):5014–20.
Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, et al. Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol. 2008;121(2):499–505.
Compliance with Ethics Guidelines
Conflict of Interest
Jason Gotlib declares the following conflicts of interest: Incyte, Inc.: receives funding to conduct a clinical trial of ruxolitinib in myelofibrosis; receives honoraria for serving on advisory boards; Pharmacyclics, Inc.: receives funding for administration of a clinical trial of ibrutinib in mastocytosis; Seattle Genetics: receives funding for conduct of a clinical trial with brentuximab vedotin in mastocytosis; Novartis, Inc.: receives funding to conduct clinical trials of midostaurin in mastocytosis and is Chair of study steering committee for the global trial of midostaurin in advanced mastocytosis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Myeloproliferative Disorders
Rights and permissions
About this article
Cite this article
Gotlib, J. Tyrosine Kinase Inhibitors and Therapeutic Antibodies in Advanced Eosinophilic Disorders and Systemic Mastocytosis. Curr Hematol Malig Rep 10, 351–361 (2015). https://doi.org/10.1007/s11899-015-0280-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-015-0280-3