Abstract
Lenalidomide is nowadays an accepted standard treatment for del(5q) MDS. In non-del(5q) disease, its role is more difficult ot define. Studies have shown that about 18 % of patients treated with a standard dose of 10 mg/day on 21 out of 28 days might achieve erythroid transfusion independence rates that last 6 months or longer. The responses to lenalidomide seem to be inversely correlated to the pre-treatment EPO level. The higher the EPO level, the lower the responses. In the absence of other cytogenetic or molecular predictive factors that allow to discern which patient benefit most from treatment, its incorporation into the treatment algorithm is dependent on the available alternatives, including erythropoietic agents, immunosupressive treatments and experimental strategies like thrombopoietin receptor agonists or the antagonists of transforming growth factor beta. Given that 90 % of responses to lenaldiomide occur within four months of treatment, patients not responding within this time frame should discontinue therapy.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–76. Important study in the treatment of del(5q) MDS patients.
Sekeres MA, Maciejewski JP, Giagounidis AA, et al. Relationship of treatment-related cytopenias and response to lenalidomide in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2008;26:5943–9.
Giagounidis A, Mufti GJ, Fenaux P, et al. Lenalidomide as a disease-modifying agent in patients with del(5q) myelodysplastic syndromes: linking mechanism of action to clinical outcomes. Ann Hematol. 2014;93:1–11.
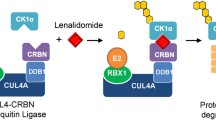
Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–9.
Fenaux P. Myelodysplastic syndromes: From pathogenesis and prognosis to treatment. Semin Hematol. 2004;41:6–12.
Neukirchen J, Lauseker M, Blum S, et al. Validation of the revised International Prognostic Scoring System (IPSS-R) in patients with myelodysplastic syndrome: A multicenter study. Leuk Res. 2014;38:57–64.
Jadersten M, Montgomery SM, Dybedal I, et al. Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood. 2005;106:803–11.
Ades L, Boehrer S, Prebet T, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113:3947–52.
Giagounidis A, Mufti GJ, Fenaux P, et al. Results of a randomized, double-blind study of romiplostim versus placebo in patients with low/intermediate-1-risk myelodysplastic syndrome and thrombocytopenia. Cancer. 2014;120:1838–46. Important data on the use of TPO-mimetics in myelodysplastic syndromes.
Angelucci E, Santini V, Di Tucci AA, et al. Deferasirox for transfusion-dependent patients with myelodysplastic syndromes: safety, efficacy, and beyond (GIMEMA MDS0306 Trial). Eur J Haematol. 2014;92:527–36.
Gattermann N, Finelli C, Della Porta M, et al. Hematologic responses to deferasirox therapy in transfusion-dependent patients with myelodysplastic syndromes. Haematologica. 2012;97:1364–71.
List AF, Baer MR, Steensma DP, et al. Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion-dependent patients with myelodysplastic syndrome. J Clin Oncol. 2012;30:2134–9.
Nolte F, Hochsmann B, Giagounidis A, et al. Results from a 1-year, open-label, single arm, multi-center trial evaluating the efficacy and safety of oral Deferasirox in patients diagnosed with low and int-1 risk myelodysplastic syndrome (MDS) and transfusion-dependent iron overload. Ann Hematol. 2013;92:191–8.
Hellstrom-Lindberg E, Gulbrandsen N, Lindberg G, et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003;120:1037–46.
Santini V. Treatment of low-risk myelodysplastic syndrome: hematopoietic growth factors erythropoietins and thrombopoietins. Semin Hematol. 2012;49:295–303.
Santibanez JF, Quintanilla M, Bernabeu C. TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2011;121:233–51.
Yu J, Shao LE, Lemas V, et al. Importance of FSH-releasing protein and inhibin in erythrodifferentiation. Nature. 1987;330:765–7.
Broxmeyer HE, Lu L, Cooper S, et al. Selective and indirect modulation of human multipotential and erythroid hematopoietic progenitor cell proliferation by recombinant human activin and inhibin. Proc Natl Acad Sci U S A. 1988;85:9052–6.
Shiozaki M, Kosaka M, Eto Y. Activin A: a commitment factor in erythroid differentiation. Biochem Biophys Res Commun. 1998;242:631–5.
Platzbecker U, Germing U, Giagounidis A et al. ACE-536 Increases Hemoglobin and Reduces Transfusion Burden in Patients with Low or Intermediate-1 Risk Myelodysplastic Syndromes (MDS): Preliminary Results from a Phase 2 Study. 2014.
Passweg JR, Giagounidis AA, Simcock M et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care--SAKK 33/99. J Clin Oncol 29: 303-309.
Sloand EM, Olnes MJ, Shenoy A, et al. Alemtuzumab treatment of intermediate-1 myelodysplasia patients is associated with sustained improvement in blood counts and cytogenetic remissions. J Clin Oncol. 2010;28:5166–73.
List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–65.
Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–76.
Giagounidis AA, Kulasekararaj A, Germing U, et al. Long-term transfusion independence in del(5q) MDS patients who discontinue lenalidomide. Leukemia. 2012;26:855–8. This manuscript can guide the clinician in the daily management of lenalidomide therapy of del(5q) patients.
Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93.
Santini V, Almeida A, Giagounidis A et al. Efficacy and Safety of Lenalidomide (LEN) Versus Placebo (PBO) in RBC-Transfusion Dependent (TD) Patients (Pts) with IPSS Low/Intermediate (Int-1)-Risk Myelodysplastic Syndromes (MDS) without Del(5q) and Unresponsive or Refractory to Erythropoiesis-Stimu…. 2014.
Santini V, Li JS, Swern AS et al. MDS-005 study: Effect oF baseline endogenous erythropoietin on rbc transfusion independence in lenalidomide-treated patients with low or intermediate-1-risk myelodysplastic syndromes without del(5q). Haematologica 2015; epub ahead of print.
Ximeri M, Galanopoulos A, Klaus M, et al. Effect of lenalidomide therapy on hematopoiesis of patients with myelodysplastic syndrome associated with chromosome 5q deletion. Haematologica. 2010;95:406–14.
McGraw KL, Basiorka AA, Johnson JO, et al. Lenalidomide induces lipid raft assembly to enhance erythropoietin receptor signaling in myelodysplastic syndrome progenitors. PLoS ONE. 2014;9:e114249.
Komrokji RS, Lancet JE, Swern AS, et al. Combined treatment with lenalidomide and epoetin alfa in lower-risk patients with myelodysplastic syndrome. Blood. 2012;120:3419–24.
Toma A, Chevret S, Kosmider O et al. A randomized study of lenalidomide (LEN) with or without EPO in RBC transfusion dependent (TD) IPSS low and int-1 (lower risk) myelodysplastic syndromes (MDS) without del 5q resistant to EPO. J Clin Oncol(Meeting Abstracts) 2013; 31: suppl 7002.
Ades L, Prebet T, Stamatoullas A, et al. Lenalidomide (LEN) Combined to Intensive Chemotherapy (IC) In AML and Higher Risk MDS with Del 5q. Results of a Phase I/II Study of the Groupe Francophone Des Myelodysplasies (GFM). ASH Annual. Meet Abstracts. 2010;116:508.
Sekeres MA, Komrokji RS, Lancet JE, et al. Final Results From the Phase 2 Continuation Study of the Lenalidomide and Azacitidine Combination in Patients with Higher-Risk Myelodysplastic Syndromes (MDS). ASH Ann Meet Abstracts. 2011;118:607.
Sekeres MA, Othus M, List AF et al. A Randomized Phase II Study of Azacitidine Combined with Lenalidomide or with Vorinostat Vs. Azacitidine Monotherapy in Higher-Risk Myelodysplastic Syndromes (MDS) and Chronic Myelomonocytic Leukemia (CMML): North American Intergroup Study SWOG S1117. 2014.
Conflict of Interest
Aristoteles Giagounidis declares no potential conflicts of interest.
Dr. Giagounidis reports personal fees from Celgene Corporation, personal fees from Novartis, and personal fees from GlaxoSmithKline.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Topical Collection on Myelodysplastic Syndromes
Rights and permissions
About this article
Cite this article
Giagounidis, A. Where Does Lenalidomide Fit in Non-del(5q) MDS?. Curr Hematol Malig Rep 10, 303–308 (2015). https://doi.org/10.1007/s11899-015-0275-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-015-0275-0