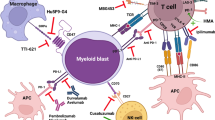
Abstract
The treatment landscape for myelodysplastic syndromes (MDS) has evolved over the last two decades, with a better understanding of the disease pathophysiology and the use of newer or combination therapies. For lower-risk MDS patients, hematopoietic growth factors have continued to be the mainstay of therapy. However, better patient selection criteria and decision tools to predict responses have made these therapies more beneficial to patients. As the range of newer drugs continues to expand in our treatment armamentarium for lower-risk MDS, questions still remain regarding the safety of these drugs with long-term use. This review will discuss the role of growth factors in MDS, focusing on dosing and combination strategies to improve responses, selecting the appropriate patient population, and recognizing the safety profile based on evidence from published literature.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of outstanding importance
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–49.
Santini V. Clinical use of erythropoietic stimulating agents in myelodysplastic syndromes. Oncologist. 2011;16(3):35–42.
Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–603.
Davidoff AJ, Weiss SR, Baer MR, Ke X, Hendrick F, Zeidan A, et al. Patterns of erythropoiesis-stimulating agent use among Medicare beneficiaries with myelodysplastic syndromes and consistency with clinical guidelines. Leuk Res. 2013;37(6):675–80.
Steensma DP, Heptinstall KV, Johnson VM, Novotny PJ, Sloan JA, Camoriano JK, et al. Common troublesome symptoms and their impact on quality of life in patients with myelodysplastic syndromes (MDS): results of a large internet-based survey. Leuk Res. 2008;32(5):691–8.
Sekeres MA, Schoonen WM, Kantarjian H, List A, Fryzek J, Paquette R, et al. Characteristics of US patients with myelodysplastic syndromes: results of six cross-sectional physician surveys. J Natl Cancer Inst. 2008;100(21):1542–51.
Sekeres MA, Maciejewski JP, List AF, Steensma DP, Artz A, Swern AS, et al. Perceptions of disease state, treatment outcomes, and prognosis among patients with myelodysplastic syndromes: results from an internet-based survey. Oncologist. 2011;16(6):904–11.
Hellström-Lindberg E, Negrin R, Stein R, Krantz S, Lindberg G, Vardiman J, et al. Erythroid response to treatment with G-CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: proposal for a predictive model. Br J Haematol. 1997;99(2):344–51.
Hellström-Lindberg E, Gulbrandsen N, Lindberg G, Ahlgren T, Dahl IM, Dybedal I, et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003;120(6):1037–46.
Sekeres MA, Fu AZ, Maciejewski JP, Golshayan AR, Kalaycio ME, Kattan MW. A decision analysis to determine the appropriate treatment for low-risk myelodysplastic syndromes. Cancer. 2007;109(6):1125–32.
Westers TM, Alhan C, Chamuleau ME, van der Vorst MJ, Eeltink C, Ossenkoppele GJ, et al. Aberrant immunophenotype of blasts in myelodysplastic syndromes is a clinically relevant biomarker in predicting response to growth factor treatment. Blood. 2010;115(9):1779–84. In this study, the authors present a novel model for predicting response to ESA using pretreatment serum erythropoietin levels and flow cytometry findings.
Park S, Kelaidi C, Sapena R, Vassilieff D, Beyne-Rauzy O, Coiteux V, et al. Early introduction of ESA in low risk MDS patients may delay the need for RBC transfusion: a retrospective analysis on 112 patients. Leuk Res. 2010;34(11):1430–6.
Terpos E, Mougiou A, Kouraklis A, Chatzivassili A, Michalis E, Giannakoulas N, et al. Prolonged administration of erythropoietin increases erythroid response rate in myelodysplastic syndromes: a phase II trial in 281 patients. Br J Haematol. 2002;118(1):174–80.
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25.
Nilsson-Ehle H, Birgegård G, Samuelsson J, Antunovic P, Astermark J, Garelius H, et al. Quality of life, physical function and MRI T2* in elderly low-risk MDS patients treated to a hemoglobin level of 120 g/l with darbepoetin alfa ± filgrastim, or erythrocyte transfusions. Eur J Haematol. 2011;87(3):244–52.
Kelaidi C, Beyne-Rauzy O, Braun T, Sapena R, Cougoul P, Adès L, et al. High response rate and improved exercise capacity and quality of life with a new regimen of darbepoetin alfa with or without filgrastim in lower-risk myelodysplastic syndromes: a phase II study by the GFM. Ann Hematol. 2013;92(5):621–31.
Greenberg PL, Sun Z, Miller KB, Bennett JM, Tallman MS, Dewald G, et al. Treatment of myelodysplastic syndromes patients with erythropoietin with or without granulocyte colony-stimulating factor: results of a prospective randomized phase III trial by the Eastern Cooperative Oncology Group (E1996). Blood. 2009;114(12):2393–400.
Casadevall N, Durieux P, Dubois S, Hemery F, Lepage E, Quarré MC, et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colony-stimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood. 2004;104(2):321–7.
Mundle S, Lefebvre P, Vekeman F, Duh MS, Rastogi R, Moyo V. An assessment of erythroid response to epoetin alpha as a single agent versus in combination with granulocyte- or granulocyte macrophage-colony-stimulating factor in myelodysplastic syndromes using a meta-analysis approach. Cancer. 2009;115(4):706–15.
Park S, Grabar S, Kelaidi C, Beyne-Rauzy O, Picard F, Bardet V, et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: the GFM experience. Blood. 2008;111(2):574–82.
Kelaidi C, Park S, Brechignac S, Mannone L, Vey N, Dombret H, et al. Treatment of myelodysplastic syndromes with 5q deletion before the lenalidomide era; the GFM experience with EPO and thalidomide. Leuk Res. 2008;32(7):1049–53.
Moyo V, Lefebvre P, Duh MS, Yektashenas B, Mundle S. Erythropoiesis-stimulating agents in the treatment of anemia in myelodysplastic syndromes: a meta-analysis. Ann Hematol. 2008;87(7):527–36.
Ross SD, Allen IE, Probst CA, Sercus B, Crean SM, Ranganathan G. Efficacy and safety of erythropoiesis-stimulating proteins in myelodysplastic syndrome: a systematic review and meta-analysis. Oncologist. 2007;12(10):1264–73.
Jädersten M, Montgomery SM, Dybedal I, Porwit-MacDonald A, Hellström-Lindberg E. Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood. 2005;106(3):803–11.
Golshayan AR, Jin T, Maciejewski J, Fu AZ, Bershadsky B, Kattan MW, et al. Efficacy of growth factors compared to other therapies for low-risk myelodysplastic syndromes. Br J Haematol. 2007;137(2):125–32.
Smith SW, Sato M, Gore SD, Baer MR, Ke X, McNally D, et al. Erythropoiesis-stimulating agents are not associated with increased risk of thrombosis in patients with myelodysplastic syndromes. Haematologica. 2012;97(1):15–20. This is one of the largest study to addresses the clinically important question of risk of thrombosis with use of ESAs in MDS patients.
Willemze R, van der Lely N, Zwierzina H, Suciu S, Solbu G, Gerhartz H, et al. A randomized phase-I/II multicenter study of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) therapy for patients with myelodysplastic syndromes and a relatively low risk of acute leukemia. EORTC Leukemia Cooperative Group. Ann Hematol. 1992;64(4):173–80.
Jakob A, Hirsch FW, Engelhardt M. Successful treatment of a patient with myelodysplastic syndrome (RAEB) with darbepoetin-alfa in combination with pegfilgrastim. Ann Hematol. 2005;84:694–5.
Greenberg P, Taylor K, Larson RA, et al. Phase III randomized multicenter trial of recombinant human G-CSF in MDS [abstract]. Blood (ASH Annual Meeting Extracts). 1993;82 Suppl 1:196a.
Schuster MW, Thompson JA, Larson RA, et al. Randomized trial of subcutaneous granulocyte-macrophage colony-stimulating factor (GM-CSF) versus observation in patients (pts) with myelodysplastic syndrome (MDS). J Cancer Res Clin Oncol. 1990;116:1079a.
Garcia-Manero G, Shan J, Faderl S, Cortes J, Ravandi F, Borthakur G, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22(3):538–43.
The Trial to Reduce Alloimmunization to Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 1997;337(26):1861–9.
Kerkhoffs JL, Eikenboom JC, van de Watering LM, van Wordragen-Vlaswinkel RJ, Wijermans PW, Brand A. The clinical impact of platelet refractoriness: correlation with bleeding and survival. Transfusion. 2008;48(9):1959–65.
Heddle Na K, J ed. Febrile Nonhemolytic transfusion reactions. In: MA P, editor. Transfusion reactions. Bethesda, MD: AABB Press; 2001.
Kuter DJ, Beeler DL, Rosenberg RD. The purification of megapoietin: a physiological regulator of megakaryocyte growth and platelet production. Proc Natl Acad Sci U S A. 1994;91(23):11104–8.
Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354(19):2034–45.
Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98(12):3241–8.
Kantarjian H, Fenaux P, Sekeres MA, Becker PS, Boruchov A, Bowen D, et al. Safety and efficacy of romiplostim in patients with lower-risk myelodysplastic syndrome and thrombocytopenia. J Clin Oncol. 2010;28(3):437–44. This is one of the first studies to evaluate the role of thrombopoietin receptor agonist, romiplostim in lower-risk MDS patients.
Fenaux P, Kantarjian H, Lyons R, et al. An open-label extension study evaluating the long-term safety and efficacy of romiplostim in thrombocytopenic patients (pts) with myelodysplastic syndromes (MDS) [abstract]. ASH Ann Meeting Abs. 2009;114:2765.
Kantarjian HM, Mufti GJ, Fenaux P, et al. Treatment with the thrombopoietin (TPO)-receptor agonist romiplostim in thrombocytopenic patients (Pts) with low or intermediate-1 (int-1) risk myelodysplastic syndrome (MDS): follow-up AML and survival results of a randomized, double-blind, placebo (PBO)-controlled study. ASH Ann Meeting Abs. 2012;120(21):421.
Wang ES, Lyons RM, Larson RA, Gandhi S, Liu D, Matei C, et al. A randomized, double-blind, placebo-controlled phase 2 study evaluating the efficacy and safety of romiplostim treatment of patients with low or intermediate-1 risk myelodysplastic syndrome receiving lenalidomide. J Hematol Oncol. 2012;29:5–71. doi:10.1186/1756-8722-5-71.
Giagounidis A, Mufti GJ, Fenaux P, Sekeres MA, Szer J, Platzbecker U, et al. Results of a randomized, double-blind study of romiplostim versus placebo in patients with low/intermediate-1-risk myelodysplastic syndrome and thrombocytopenia. Cancer. 2014;120(12):1838–46.
Greenberg PL, Garcia-Manero G, Moore M, Damon L, Roboz G, Hu K, et al. A randomized controlled trial of romiplostim in patients with low- or intermediate-risk myelodysplastic syndrome receiving decitabine. Leuk Lymphoma. 2013;54(2):321–8.
Kantarjian HM, Giles FJ, Greenberg PL, Paquette RL, Wang ES, Gabrilove JL, et al. Phase 2 study of romiplostim in patients with low- or intermediate-risk myelodysplastic syndrome receiving azacitidine therapy. Blood. 2010;116(17):3163–70.
Esther Oliva, Valeria Santini, Gina Zini, Giuseppe Palumbo, Antonella Poloni, Agostino Cortelezzi, Francesco Rodeghiero, Maria Teresa Voso, Alfredo Molteni, Grazia Sanpaolo, Anna Marina Liberati, Fortunato Morabito, Enrico Balleari, Stefana Impera, Flavia Salvi, Maria Antonietta Aloe Spiriti, Antonio Marino, Filippo Rodà, Caterina Alati, Francesca Ronco, Francesco Di Raimondo, Pietro Leoni, Giuliana Alimena, Giuseppe Fioritoni, Roberto Latagliata, Francesco Nobile. Eltrombopag for the treatment of thrombocytopenia of low and intermediate-1 IPSS risk myelodysplastic syndromes: results of a prospective, randomized, trial. EHA 2013 Congress Abstract S1110 (Oral Presentation).
Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood. 2009;114:3748–56.
Luo SS, Ogata K, Yokose N, Kato T, Dan K. Effect of thrombopoietin on proliferation of blasts from patients with myelodysplastic syndromes. Stem Cells. 2000;18:112–9.
Boiocchi L, Orazi A, Ghanima W, et al. Thrombopoietin receptor agonist therapy in primary immune thrombocytopenia is associated with bone marrow hypercellularity and mild reticulin fibrosis but not other stromal abnormalities. Mod Pathol. 2012;25:65–74.
Montero AJ, Estrov Z, Freireich EJ, Khouri IF, Koller CA, Kurzrock R. Phase II study of low-dose interleukin-11 in patients with myelodysplastic syndrome. Leuk Lymphoma. 2006;47(10):2049–54.
Wu HH, Talpaz M, Champlin RE, Pilat SR, Kurzrock R. Sequential interleukin 3 and granulocyte-macrophage-colony stimulating factor therapy in patients with bone marrow failure with long-term follow-up of responses. Cancer. 2003;98(11):2410–9.
Gordon MS, Nemunaitis J, Hoffman R, et al. A phase I trial of recombinant human interleukin-6 in patients with myelodysplastic syndromes and thrombocytopenia. Blood. 1995;85(11):3066–76.
Conflict of Interest
Dr. Aakriti Pandita and Dr. Sudipto Mukherjee each declare no potential conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandita, A., Mukherjee, S. Resuscitating a Dying Marrow: the Role of Hematopoietic Growth Factors. Curr Hematol Malig Rep 9, 412–420 (2014). https://doi.org/10.1007/s11899-014-0236-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-014-0236-z