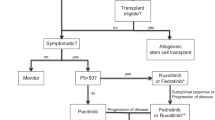
Abstract
The landscape of therapy for myelofibrosis (MF) is evolving at a pace not previously seen for this clonal myeloproliferative neoplasm. The discovery of the JAK2 V617F mutation in 2005 has led to the rapid development of therapy specifically developed for afflicted MF patients. Indeed, the successful phase III studies of ruxolitinib demonstrating improved symptomatic burden, splenomegaly and survival led to the first approved myelofibrosis drug in the United States and Europe. Multiple additional JAK2 inhibitors are currently in or nearing phase III testing, including SAR302503 (fedratinib), SB1518 (pacritinib) and CYT387 (momelotinib), seeking to offer incremental benefits to ruxolitinib in regards to cytopenias or other disease features. In parallel, phase III testing of pomalidomide is ongoing, with the goal of solidifying the role of immunomodulatory therapy in MF-associated anemia. Multiple single agents strategies are ongoing with histone deacetylase inhibitors, hedgehog inhibitors and hypomethylation agents. Incremental advances are further sought, either in additive or synergistic fashion, from combination strategies of ruxolitinib with multiple different approaches ranging from allogeneic stem cell transplant to current therapies mitigating anemia and further impacting the bone marrow microenvironment or histology. Transitioning from a pre-2011 era devoid of approved MF therapies to one of multiple agents that target not only disease course but symptomatic burden has indeed changed the platform from which MF providers are able to launch individualized treatment plans. In this article, we discuss the diagnostic and therapeutic milestones achieved through MF research and review the emerging pharmacologic agents on the treatment horizon.




Similar content being viewed by others
References
Papers of particular interest have been highlighted as: •• Of major importance
Mesa RA, Verstovsek S, Cervantes F, Barosi G, Reilly JT, Dupriez B et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res. 2007.
•• James C, Ugo V, Casadevall N, Constantinescu SN, Vainchenker W. A JAK2 mutation in myeloproliferative disorders: Pathogenesis and therapeutic and scientific prospects. Trends Mol Med. 2005;11(12):546–54. The first description of JAK2-V617F in MPNs, a watershed both in therapeutic targeting and well as pathogenetic insight.
Kralovics R, Passamonti F, Buser AS, Soon-Siong T, Tiedt R, Passweg JR, et al. A gain of function mutation in Jak2 is frequently found in patients with myeloproliferative disorders. New Engl J Med. 2005;352:1779–90.
Abdel-Wahab O, Tefferi A, Levine RL. Role of TET2 and ASXL1 mutations in the pathogenesis of myeloproliferative neoplasms. Hematol Oncol Clin North Am. 2012;26(5):1053–64. doi:10.1016/j.hoc.2012.07.006.
Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360(22):2289–301. doi:10.1056/NEJMoa0810069.
Oh ST, Simonds EF, Jones C, Hale MB, Goltsev Y, Gibbs Jr KD, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010;116(6):988–92. doi:10.1182/blood-2010-02-270108.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270.
Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nat Rev Cancer. 2012;12(9):599–612. doi:10.1038/nrc3343.
Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24(6):1128–38. doi:10.1038/leu.2010.69.
Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;118(7):1723–35. doi:10.1182/blood-2011-02-292102.
Zhang SJ, Abdel-Wahab O. Disordered epigenetic regulation in the pathophysiology of myeloproliferative neoplasms. Curr Hematol Malig Rep. 2012;7(1):34–42. doi:10.1007/s11899-011-0105-y.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the WHO classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009. doi:10.1182/blood-2009-03-209262.
Mesa RA, Shields A, Hare T, Erickson-Viitanen S, Sun W, Sarlis NJ, et al. Progressive burden of myelofibrosis in untreated patients: Assessment of patient-reported outcomes in patients randomized to placebo in the COMFORT-I study. Leuk Res. 2013. doi:10.1016/j.leukres.2013.04.017.
Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–901. doi:10.1182/blood-2008-07-170449.
Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: Prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–103. doi:10.1200/JCO.2012.42.3863.
Barbui T, Carobbio A, Cervantes F, Vannucchi AM, Guglielmelli P, Antonioli E, et al. Thrombosis in primary myelofibrosis: Incidence and risk factors. Blood. 2010;115(4):778–82. doi:10.1182/blood-2009-08-238956.
Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. The myeloproliferative neoplasm symptom assessment form (MPN-SAF): International prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–8. doi:10.1182/blood-2011-01-328955.
Geyer HL, Dueck AC, Emanuel RM, Kiladjian J-J, Slot S, Zweegman S, et al. The myelofibrosis symptom burden (MF-SB): An international phenotypic cluster analysis of 329 patients. ASH Annual Meeting Abstracts. 2012;120(21):1731.
Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: A study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–8. doi:10.1182/blood-2009-09-245837.
Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: A refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392–7.
Mesa RA. The evolving treatment paradigm in myelofibrosis. Leuk Lymphoma. 2013;54(2):242–51. doi:10.3109/10428194.2012.710905.
Kroger N, Holler E, Kobbe G, Bornhauser M, Schwerdtfeger R, Baurmann H, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the chronic leukemia working party of the European group for blood and marrow transplantation. Blood. 2009;114(26):5264–70. doi:10.1182/blood-2009-07-234880.
Fauble V, Leis J, Mesa R. Allogeneic stem cell transplant for myelofibrosis patients over age 60: likely impact of the JAK2 inhibitors. Leukemia. 2012(Supplement 2012):S2–S7
Cervantes F, Alvarez-Larran A, Domingo A, Arellano-Rodrigo E, Montserrat E. Efficacy and tolerability of danazol as a treatment for the anaemia of myelofibrosis with myeloid metaplasia: Long-term results in 30 patients. Br J Haematol. 2005;129(6):771–5.
Tsiara SN, Chaidos A, Bourantas LK, Kapsali HD, Bourantas KL. Recombinant human erythropoietin for the treatment of anaemia in patients with chronic idiopathic myelofibrosis. Acta Haematol. 2007;117(3):156–61. doi:10.1159/000097463.
Huang J, Tefferi A. Erythropoiesis stimulating agents have limited therapeutic activity in transfusion-dependent patients with primary myelofibrosis regardless of serum erythropoietin level. Eur J Haematol. 2009;83(2):154–5. doi:10.1111/j.1600-0609.2009.01266.x.
Thapaliya P, Tefferi A, Pardanani A, Steensma DP, Camoriano J, Wu W, et al. International working group for myelofibrosis research and treatment response assessment and long-term follow-up of 50 myelofibrosis patients treated with thalidomide-prednisone based regimens. Am J Hematol. 2011;86(1):96–8. doi:10.1002/ajh.21892.
Martinez-Trillos A, Gaya A, Maffioli M, Arellano-Rodrigo E, Calvo X, Diaz-Beya M, et al. Efficacy and tolerability of hydroxyurea in the treatment of the hyperproliferative manifestations of myelofibrosis: Results in 40 patients. Ann Hematol. 2010;89(12):1233–7.
•• Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–98. doi:10.1056/NEJMoa1110556. First randomized phase III trial of a JAK2 inhibitor vs. best available therapy in myelofibrosis.
Faoro L, Mesa R, Gertz MA, Tefferi A. Long-term analysis of the palliative benefit of 2-Chlorodeoxyadenosine (2-CdA) for myelofibrosis with myeloid metaplasia. Am J Clin Oncol. 2004.
Mesa RA, Nagorney DS, Schwager S, Allred J, Tefferi A. Palliative goals, patient selection, and perioperative platelet management: outcomes and lessons from 3 decades of splenectomy for myelofibrosis with myeloid metaplasia at the Mayo Clinic. Cancer. 2006;107(2):361–70.
Elliott MA, Chen MG, Silverstein MN, Tefferi A. Splenic irradiation for symptomatic splenomegaly associated with myelofibrosis with myeloid metaplasia. Br J Haematol. 1998;103(2):505–11.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 Inhibitor, in Myelofibrosis. New Engl J Med. 2010;363(12):1117–27. doi:10.1056/NEJMoa1002028.
Verstovsek S, Kantarjian HM, Estrov Z, Cortes JE, Thomas DA, Kadia T, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: Survival advantage in comparison to matched historical controls. Blood. 2012;120(6):1202–9. doi:10.1182/blood-2012-02-414631.
Kvasnicka H-M, Thiele J, Bueso-Ramos CE, Hou K, Cortes JE, Kantarjian HM. Exploratory analysis of the effect of ruxolitinib on bone marrow morphology in patients with myelofibrosis. J Clin Oncol. 2013;31:7030.
•• Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi:10.1056/NEJMoa1110557. First randomized trial of JAK2 inhibitor vs. placebo, demonstrating a survival advantage in myelofibrosis.
Mesa RA, Gotlib J, Gupta V, Catalano JV, Deininger MW, Shields AL, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2013;31(10):1285–92. doi:10.1200/JCO.2012.44.4489.
Harrison CN, Mesa RA, Kiladjian JJ, Al-Ali HK, Gisslinger H, Knoops L, et al. Health-related quality of life and symptoms in patients with myelofibrosis treated with ruxolitinib versus best available therapy. Br J Haematol. 2013. doi:10.1111/bjh.12375.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. The clinical benefit of ruxolitinib across patient subgroups: analysis of a placebo-controlled, Phase III study in patients with myelofibrosis. Br J Haematol. 2013;161(4):508–16. doi:10.1111/bjh.12274.
Vannucchi A, Cervantes F, Niederwieser D, Sirulnik A, Stalbovskaya V, McQuitty M et al. Long-term outcomes from a phase 3 study comparing ruxolitinib with best available therapy (BAT) for the treatment of myelofibrosis (MF): A 3-YEAR update of COMFORT-II. Haematologica. 2013; Suppl:a5828.
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29(7):789–96.
Animesh Dev Pardanani CHMJ, Gabrail NY, et al. Updated results from a randomized phase II dose-ranging study of the JAK2-selective inhibitor SAR302503 in patients with myelofibrosis (MF). J Clin Oncol. 2013;31:7109.
Deeg HJOO, Scott BL, et al. Phase II study of SB1518, an orally available novel Jak2 inhibitor, in patients with myelofibrosis. J Clin Oncol. 2011;29:6515.
Komrokji RS, Wadleigh M, Seymour JF, Roberts AW, To LB, Zhu HJ, et al. Results of a Phase 2 Study of Pacritinib (SB1518), a Novel Oral JAK2 Inhibitor, In Patients with Primary, Post-Polycythemia Vera, and Post-Essential Thrombocythemia Myelofibrosis. ASH Ann Meet Abs. 2011;118(21):282.
Pardanani A, Laborde RR, Lasho TL, Finke C, Begna K, Al-Kali A, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27(6):1322–7. doi:10.1038/leu.2013.71.
Tefferi A, Verstovsek S, Barosi G, Passamonti F, Roboz GJ, Gisslinger H et al. Pomalidomide Is Active in the Treatment of Anemia Associated With Myelofibrosis. J Clin Oncol. 2009.
Mesa RA, Pardanani AD, Hussein K, Wu W, Schwager S, Litzow MR, et al. Phase1/-2 study of Pomalidomide in myelofibrosis. Am J Hematol. 2010;85(2):129–30. doi:10.1002/ajh.21598.
Deangelo DJ, Mesa RA, Fiskus W, Tefferi A, Paley C, Wadleigh M, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol. 2013. doi:10.1111/bjh.12384.
Rambaldi A, Dellacasa CM, Finazzi G, Carobbio A, Ferrari ML, Guglielmelli P, et al. A pilot study of the histone-deacetylase inhibitor givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol. 2010;150(4):446–55. doi:10.1111/j.1365-2141.2010.08266.x.
Guglielmelli P, Barosi G, Rambaldi A, Marchioli R, Masciulli A, Tozzi L, et al. Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood. 2011;118(8):2069–76. doi:10.1182/blood-2011-01-330563.
Constantinescu SN, Vainchenker W. Small-molecule inhibitors in myeloproliferative neoplasms: Are we aiming for the right targets? Hematol Am Soc Hematol Educ Program. 2012;2012:553–60. doi:10.1182/asheducation-2012.1.553.
Compliance with Ethics Guidelines
Conflict of Interest
Krisstina Gowin, Robyn Emanuel, and Holly Geyer declare that they have no conflict of interest.
Ruben A. Mesa has received payment for consulting from Novartis, and research support from Sanofi, Incyte, Genentech, Lilly, Gilead, CTI, and Celgene.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gowin, K., Emanuel, R., Geyer, H. et al. The New Landscape of Therapy for Myelofibrosis. Curr Hematol Malig Rep 8, 325–332 (2013). https://doi.org/10.1007/s11899-013-0178-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-013-0178-x