Abstract
Purpose of Review
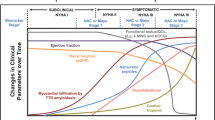
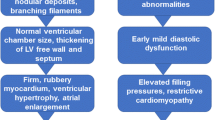
Transthyretin cardiac amyloidosis (ATTR-CM) is an infiltrative cardiomyopathy and an increasingly recognized cause of morbidity and mortality. There remains substantial delay between initial symptoms and diagnosis. With the recent emergence of various targeted therapies proven to reduce morbidity and mortality, there is an imperative to diagnose subclinical disease. Biomarkers may be well-suited for this role.
Recent Findings
Conventional markers of heart failure, such as natriuretic peptides and cardiac troponins, and estimated glomerular filtration rate are associated with risk in ATTR-CM. Circulating transthyretin (TTR) levels parallel TTR kinetic stability, correlate with disease severity, and may serve as indirect markers of ATTR-CM disease activity and response to targeted treatment. There is also growing evidence for the correlation of TTR to retinol-binding protein 4, a biomarker which independently associates with this disease. The rate-limiting step for ATTR pathogenesis is dissociation of the TTR homotetramer, which may be quantified using subunit exchange to allow for early risk assessment, prognostication, and assessment of treatment response. The protein species that result from the dissociation and misfolding of TTR are known as nonnative transthyretin (NNTTR). NNTTR is quantifiable via peptide probes and is a specific biomarker whose reduction is positively correlated with improvement in neuropathic ATTR amyloidosis. Neurofilament light chain (NfL) is released into the blood after axonal damage and correlates with neuropathic ATTR amyloidosis, but its clinical use in ATTR-CM is uncertain.
Summary
Conventional markers of heart failure, transthyretin, retinol-binding protein 4, transthyretin kinetic stability, nonnative transthyretin, peptide probes, and neurofilament light chain have potential as biomarkers to enable early, subclinical diagnosis in patients with transthyretin cardiac amyloidosis.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hanson JLS, Arvanitis M, Koch CM, Berk JL, Ruberg FL, Prokaeva T, et al. Use of serum transthyretin as a prognostic indicator and predictor of outcome in cardiac amyloid disease associated with wild-type transthyretin. Circ Heart Fail. 2018;11(2):e004000.
Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203–12.
Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098.
Lane T, Fontana M, Martinez-Naharro A, Quarta CC, Whelan CJ, Petrie A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26.
Coelho T, Maurer MS, Suhr OB. THAOS—the transthyretin amyloidosis outcomes survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63–76.
Damy T, Adams D, Bridoux F, Grateau G, Plante-Bordeneuve V, Ghiron Y, et al. Amyloidosis from the patient perspective: the French daily impact of amyloidosis study. Amyloid. 2022:1–10.
•• Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–16. This study is a major clinical trial demonstrating the clinical efficacy of transythretin-stabilizing therapies to improve clinical outcomes.
Plante-Bordeneuve V, Gorram F, Salhi H, Nordine T, Ayache SS, Le Corvoisier P, et al. Long-term treatment of transthyretin familial amyloid polyneuropathy with tafamidis: a clinical and neurophysiological study. J Neurol. 2017;264(2):268–76.
Lozeron P, Theaudin M, Mincheva Z, Ducot B, Lacroix C, Adams D, et al. Effect on disability and safety of Tafamidis in late onset of Met30 transthyretin familial amyloid polyneuropathy. Eur J Neurol. 2013;20(12):1539–45.
Castiglione V, Franzini M, Aimo A, Carecci A, Lombardi CM, Passino C, et al. Use of biomarkers to diagnose and manage cardiac amyloidosis. Eur J Heart Fail. 2021;23(2):217–30.
Hall C. NT-ProBNP: the mechanism behind the marker. J Card Fail. 2005;11(5 Suppl):S81–3.
York MK, Gupta DK, Reynolds CF, Farber-Eger E, Wells QS, Bachmann KN, et al. B-type natriuretic peptide levels and mortality in patients with and without heart failure. J Am Coll Cardiol. 2018;71(19):2079–88.
Lehrke S, Steen H, Kristen AV, Merten C, Lossnitzer D, Dengler TJ, et al. Serum levels of NT-proBNP as surrogate for cardiac amyloid burden: new evidence from gadolinium-enhanced cardiac magnetic resonance imaging in patients with amyloidosis. Amyloid. 2009;16(4):187–95.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618–51.
Takashio S, Yamamuro M, Izumiya Y, Hirakawa K, Marume K, Yamamoto M, et al. Diagnostic utility of cardiac troponin T level in patients with cardiac amyloidosis. ESC Heart Fail. 2018;5(1):27–35.
Dubin RF, Li Y, He J, Jaar BG, Kallem R, Lash JP, et al. Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: a cross-sectional study in the chronic renal insufficiency cohort (CRIC). BMC Nephrol. 2013;14:229.
Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–20.
Garg P, Morris P, Fazlanie AL, Vijayan S, Dancso B, Dastidar AG, et al. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med. 2017;12(2):147–55.
Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39(30):2799–806.
• Falk RH, Haddad M, Walker CR, Dorbala S, Cuddy SAM. Effect of tafamidis on serum transthyretin levels in non-trial patients with transthyretin amyloid cardiomyopathy. JACC CardioOncol. 2021;3(4):580–6. This study associates the change in transthyretin levels with a response to therapy in a non-trial cohort, offering external validity to observations of previous controlled trials.
Garcia-Pavia P, Bengel F, Brito D, Damy T, Duca F, Dorbala S, et al. Expert consensus on the monitoring of transthyretin amyloid cardiomyopathy. Eur J Heart Fail. 2021;23(6):895–905.
Maleszewski JJ. Cardiac amyloidosis: pathology, nomenclature, and typing. Cardiovasc Pathol. 2015;24(6):343–50.
Myron Johnson A, Merlini G, Sheldon J, Ichihara K, Scientific Division Committee on Plasma Proteins IFoCC, Laboratory M. Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419–26.
Dickson PW, Howlett GJ, Schreiber G. Metabolism of prealbumin in rats and changes induced by acute inflammation. Eur J Biochem. 1982;129(2):289–93.
Oppehneimer JH, Surks MI, Bernstein G, Smity JC. Metabolism of iodine-131—labeled thyroxine-binding prealbumin in man. Science. 1965;149(3685):748–50.
Ingenbleek Y, Young V. Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr. 1994;14:495–533.
Ritchie RF, Navolotskaia O. Serum proteins in clinical medicine: American Association for Clinical Chemistry; 1996.
Greve AM, Christoffersen M, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Association of low plasma transthyretin concentration with risk of heart failure in the general population. JAMA Cardiol. 2021;6(3):258–66.
Hornstrup LS, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Genetic stabilization of transthyretin, cerebrovascular disease, and life expectancy. Arterioscler Thromb Vasc Biol. 2013;33(6):1441–7.
Buxbaum J, Anan I, Suhr O. Serum transthyretin levels in Swedish TTR V30M carriers. Amyloid. 2010;17(2):83–5.
Buxbaum J, Koziol J, Connors LH. Serum transthyretin levels in senile systemic amyloidosis: effects of age, gender and ethnicity. Amyloid. 2008;15(4):255–61.
•• Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. This study is a major clinical trial demonstrating the reduction of serum transthyretin in patients treated with therapies that silence hepatic translation of transthyretin.
• Gamino DTS, De Los SJ, Helmke S, Guadalupe S, Maurer M. Tafamidis increases serum TTR (prealbumin) levels in both ATTRh and ATTRwt cardiac amyloidosis. J Cardiac Fail. 2019;25(8):S21. This study associates changes in transthyretin levels with a response to therapy, outlining the potential for transthyretin to serve as a surrogate for treatment efficacy.
Judge DP, Heitner SB, Falk RH, Maurer MS, Shah SJ, Witteles RM, et al. Transthyretin stabilization by AG10 in symptomatic transthyretin amyloid cardiomyopathy. J Am Coll Cardiol. 2019;74(3):285–95.
Hyung SJ, Deroo S, Robinson CV. Retinol and retinol-binding protein stabilize transthyretin via formation of retinol transport complex. ACS Chem Biol. 2010;5(12):1137–46.
Noy N, Li L, Abola MV, Berger NA. Is retinol binding protein 4 a link between adiposity and cancer? Horm Mol Biol Clin Investig. 2015;23(2):39–46.
Santos D, Coelho T, Alves-Ferreira M, Sequeiros J, Mendonca D, Alonso I, et al. variants in RBP4 and AR genes modulate age at onset in familial amyloid polyneuropathy (FAP ATTRV30M). Eur J Hum Genet. 2016;24(5):756–60.
Suhr OB, Coelho T, Buades J, Pouget J, Conceicao I, Berk J, et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis. 2015;10:109.
Arvanitis M, Simon S, Chan G, Fine D, Beardsley P, LaValley M, et al. Retinol binding protein 4 (RBP4) concentration identifies V122I transthyretin cardiac amyloidosis. Amyloid. 2017;24(sup1):120–1.
Arvanitis M, Koch CM, Chan GG, Torres-Arancivia C, LaValley MP, Jacobson DR, et al. Identification of transthyretin cardiac amyloidosis using serum retinol-binding protein 4 and a clinical prediction model. JAMA Cardiol. 2017;2(3):305–13.
Kelly JW. Mechanisms of amyloidogenesis. Nat Struct Biol. 2000;7(10):824–6.
Hammarstrom P, Jiang X, Hurshman AR, Powers ET, Kelly JW. Sequence-dependent denaturation energetics: a major determinant in amyloid disease diversity. Proc Natl Acad Sci U S A. 2002;99(Suppl 4):16427–32.
Jiang X, Labaudiniere R, Buxbaum JN, Monteiro C, Novais M, Coelho T, et al. A circulating, disease-specific, mechanism-linked biomarker for ATTR polyneuropathy diagnosis and response to therapy prediction. Proc Natl Acad Sci U S A. 2021;118(9).
Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109(24):9629–34.
Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13(4):236–49.
Rappley I, Monteiro C, Novais M, Baranczak A, Solis G, Wiseman RL, et al. Quantification of transthyretin kinetic stability in human plasma using subunit exchange. Biochemistry. 2014;53(12):1993–2006.
Nelson LT, Paxman RJ, Xu J, Webb B, Powers ET, Kelly JW. Blinded potency comparison of transthyretin kinetic stabilisers by subunit exchange in human plasma. Amyloid. 2021;28(1):24–9.
Childers MC, Daggett V. Edge strand dissociation and conformational changes in transthyretin under amyloidogenic conditions. Biophys J. 2020;119(10):1995–2009.
Pires RH, Saraiva MJ, Damas AM, Kellermayer MS. Force spectroscopy reveals the presence of structurally modified dimers in transthyretin amyloid annular oligomers. J Mol Recognit. 2017;30(3).
Schonhoft JD, Monteiro C, Plate L, Eisele YS, Kelly JM, Boland D, et al. Peptide probes detect misfolded transthyretin oligomers in plasma of hereditary amyloidosis patients. Sci Transl Med. 2017;9(407).
Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A. 1987;84(10):3472–6.
Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003;987(1):25–31.
Skillback T, Farahmand B, Bartlett JW, Rosen C, Mattsson N, Nagga K, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83(21):1945–53.
Olsson B, Portelius E, Cullen NC, Sandelius A, Zetterberg H, Andreasson U, et al. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol. 2019;76(3):318–25.
Luigetti M, Di Paolantonio A, Guglielmino V, Romano A, Rossi S, Sabino A, et al. Neurofilament light chain as a disease severity biomarker in ATTRv: data from a single-centre experience. Neurol Sci. 2022.
Kapoor M, Foiani M, Heslegrave A, Zetterberg H, Lunn MP, Malaspina A, et al. Plasma neurofilament light chain concentration is increased and correlates with the severity of neuropathy in hereditary transthyretin amyloidosis. J Peripher Nerv Syst. 2019;24(4):314–9.
Ticau S, Sridharan GV, Tsour S, Cantley WL, Chan A, Gilbert JA, et al. Neurofilament light chain as a biomarker of hereditary transthyretin-mediated amyloidosis. Neurology. 2021;96(3):e412–22.
Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12(9):e006075.
Kagan J, Cabanero M, Wieczorek R, Pincus M. Can serum free light chains be used for the early diagnosis of monoclonal immunoglobulin-secreting B-cell and plasma-cell diseases? Fed Pract. 2016;33(Suppl 5):36S-S39.
Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–12.
Phull P, Sanchorawala V, Connors LH, Doros G, Ruberg FL, Berk JL, et al. Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR). Amyloid. 2018;25(1):62–7.
Hoffman JE, Sultan MB, Gundapaneni B, Witteles R. Free light-chain levels in patients with transthyretin amyloid cardiomyopathy in Attr-ACT. blood. 2021;138(Supplement 1):3787.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Justin Grodin received research funding from the Texas Health Resources Clinical Scholarship and the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL160892 and receives consulting income from Pfizer and Eidos Therapeutics. Caleb Hood declares no conflict of interest. Nicholas Hendren declares no conflict of interest. Rose Pedretti declares no conflict of interest. Lorena Saelices declares no conflict of interest. Lori Roth has done consulting work with Eidos Therapeutics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomarkers of Heart Failure
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hood, C.J., Hendren, N.S., Pedretti, R. et al. Update on Disease-Specific Biomarkers in Transthyretin Cardiac Amyloidosis. Curr Heart Fail Rep 19, 356–363 (2022). https://doi.org/10.1007/s11897-022-00570-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-022-00570-1