Abstract
Purpose of Review
This review aims to summarize the renal effects of sodium-glucose transporter-2 (SGLT-2) inhibitors and their potential implications in heart failure pathophysiology.
Recent Findings
In patients with diabetes and established atherosclerosis, the SGLT-2 inhibitor empagliflozin versus placebo significantly reduced the rate of heart failure admissions with 35%. Moreover, empagliflozin slowed kidney disease progression and reduced the need for renal replacement therapy.
Summary
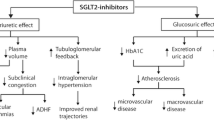
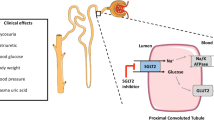
SGLT-2 inhibitors inhibit proximal tubular sodium and chloride reabsorption, leading to increased nephron flux throughout the distal renal tubules, most notably at the level of the macula densa. Afferent arteriolar vasoconstriction is promoted through tubulo-glomerular feedback and reduces glomerular capillary hydrostatic pressure, relieving podocyte stress and explaining renal preservation. Further, plasma volume is contracted and natriuresis promoted without inducing neurohumoral activation. Finally, SGLT-2 inhibitors may improve endothelial function and energy metabolism efficiency. Together, these promising features place them as a potential novel treatment for heart failure.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102(2):203–10.
Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572–80.
Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60(12):1031–42.
Cruz DN, Schmidt-Ott KM, Vescovo G, House AA, Kellum JA, Ronco C, et al. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: workgroup statements from the eleventh consensus conference of the acute dialysis quality initiative (ADQI). Contrib Nephrol. 2013;182:117–36.
Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62(6):485–95.
• Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. The only trial powered for cardiovascular end-points evaluation with a sodium-glucose transporter-2 inhibitor showing an unprecedented 38% reduction in cardiovascular mortality.
•• Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34. Subanalysis of the EMPA-REG trial with empagliflozin (reference 6) clearly demonstrating the renoprotective effect of empagliflozin in diabetes.
Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1(2):140–51.
Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16(5):457–66.
Freitas HS, Anhe GF, Melo KF, Okamoto MM, Oliveira-Souza M, Bordin S, et al. Na(+)-glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology. 2008;149(2):717–24.
•• Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–97. Insightful study demonstrating that the sodium-glucose transporter-2 inhibitor empagliflozin was able to reverse glomerular hyperfiltration in diabetes.
Calado J, Sznajer Y, Metzger D, Rita A, Hogan MC, Kattamis A, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874–9.
Elsas LJ, Busse D, Rosenberg LE. Autosomal recessive inheritance of renal glycosuria. Metabolism. 1971;20(10):968–75.
Elsas LJ, Rosenberg LE. Familial renal glycosuria: a genetic reappraisal of hexose transport by kidney and intestine. J Clin Invest. 1969;48(10):1845–54.
van den Heuvel LP, Assink K, Willemsen M, Monnens L. Autosomal recessive renal glucosuria attributable to a mutation in the sodium glucose cotransporter (SGLT2). Hum Genet. 2002;111(6):544–7.
Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, et al. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol. 2003;14(11):2873–82.
Verbrugge FH, Grieten L, Mullens W. Management of the cardiorenal syndrome in decompensated heart failure. Cardiorenal Med. 2014;4(3–4):176–88.
Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. The podocyte’s response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2013;304(4):F333–47.
Anderson S, Brenner BM. The role of intraglomerular pressure in the initiation and progression of renal disease. J Hypertens Suppl. 1986;4(5):S236–8.
Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80(3):282–7.
Braam B, Koomans HA. Renal responses to antagonism of the renin-angiotensin system. Curr Opin Nephrol Hypertens. 1996;5(1):89–96.
Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341(8):577–85.
Carmines PK, Inscho EW, Gensure RC. Arterial pressure effects on preglomerular microvasculature of juxtamedullary nephrons. Am J Phys. 1990;258(1 Pt 2):F94–102.
Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Phys. 1998;274(2 Pt 2):R263–79.
• Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WH, et al. The kidney in congestive heart failure: ‘are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur J Heart Fail. 2014;16(2):133–42. This review on renal sodium handling in heart failure comprehensively explains the rationale behind inhibition of proximal tubular sodium reabsorption in heart failure which is a major effect of sodium-glucose transporter-2 inhibitors.
• Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–72. This review comprehensively summarizes the effects of sodium-glucose transporter-2 inhibitors on both heart and kidneys.
McKie PM, Schirger JA, Costello-Boerrigter LC, Benike SL, Harstad LK, Bailey KR, et al. Impaired natriuretic and renal endocrine response to acute volume expansion in pre-clinical systolic and diastolic dysfunction. J Am Coll Cardiol. 2011;58(20):2095–103.
Verbrugge FH, Nijst P, Dupont M, Penders J, Tang WH, Mullens W. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ Heart Fail. 2014;7(5):766–72.
Miller WL, Mullan BP. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: role for blood volume quantitation. JACC Heart Fail. 2014;2(3):298–305.
Mentz RJ, Stevens SR, DeVore AD, Lala A, Vader JM, AbouEzzeddine OF, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail. 2015;3(2):97–107.
Verbrugge FH, Tang WH, Mullens W. Renin-angiotensin-aldosterone system activation during decongestion in acute heart failure: friend or foe? JACC Heart Fail. 2015;3(2):108–11.
Verbrugge FH, Steels P, Grieten L, Nijst P, Tang WH, Mullens W. Hyponatremia in acute decompensated heart failure: depletion versus dilution. J Am Coll Cardiol. 2015;65(5):480–92.
•• Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–62. Important study highlighting that plasma volume is decreased after treatment with the sodium-glucose transporter-2 inhibitor dapagliflozin in diabetes.
Cherney DZ, Scholey JW, Jiang S, Har R, Lai V, Sochett EB, et al. The effect of direct renin inhibition alone and in combination with ACE inhibition on endothelial function, arterial stiffness, and renal function in type 1 diabetes. Diabetes Care. 2012;35(11):2324–30.
Cheungpasitporn W, Thongprayoon C, Chiasakul T, Korpaisarn S, Erickson SB. Renin-angiotensin system inhibitors linked to anemia: a systematic review and meta-analysis. QJM. 2015;108(11):879–84.
Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122(3):265–72.
van der Meer P, Postmus D, Ponikowski P, Cleland JG, O'Connor CM, Cotter G, et al. The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. J Am Coll Cardiol. 2013;61(19):1973–81.
Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol. 2013;62(6):516–24.
Vaduganathan M, Greene SJ, Fonarow GC, Voors AA, Butler J, Gheorghiade M. Hemoconcentration-guided diuresis in heart failure. Am J Med. 2014;127(12):1154–9.
• Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39(7):1108–14. Interesting review explaining the hypothesis that sodium-glucose transporter-2 inhibitors improve energy metabolism by stimulating β-hydroxybutyrate oxidation as an efficient energy fuel.
Zahedi K, Barone S, Xu J, Soleimani M. Potentiation of the effect of thiazide derivatives by carbonic anhydrase inhibitors: molecular mechanisms and potential clinical implications. PLoS One. 2013;8(11):e79327.
Imai T, Akimoto T, Ito C, Masuda T, Nagata D. Management of diabetes associated with nephrotic syndrome: therapeutic potential of dapagliflozin for protracted volume retention. Drug Target Insights. 2015;9:29–31.
Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9(10):1064–9.
Verbrugge FH, Dupont M, Bertrand PB, Nijst P, Penders J, Dens J, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol. 2015;70(3):265–73.
Verbrugge FH, Mullens W, Tang WH. Management of cardio-renal syndrome and diuretic resistance. Curr Treat Options Cardiovasc Med. 2016;18(2):11.
Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60(16):1455–69.
Mackenzie IS, McEniery CM, Dhakam Z, Brown MJ, Cockcroft JR, Wilkinson IB. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54(2):409–13.
Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, Tang WH, et al. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol. 2015;65(4):378–88.
Nishimura R, Tanaka Y, Koiwai K, Inoue K, Hach T, Salsali A, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11.
Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24-week, randomized, double-blind, placebo-controlled. Phase III study Expert Opin Pharmacother. 2014;15(11):1501–15.
Balasse EO, Fery F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5(3):247–70.
Natali A, Buzzigoli G, Taddei S, Santoro D, Cerri M, Pedrinelli R, et al. Effects of insulin on hemodynamics and metabolism in human forearm. Diabetes. 1990;39(4):490–500.
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Frederik H. Verbrugge, Pieter Martens, and Wilfried Mullens declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pathophysiology of Myocardial Failure
Rights and permissions
About this article
Cite this article
Verbrugge, F.H., Martens, P. & Mullens, W. SGLT-2 Inhibitors in Heart Failure: Implications for the Kidneys. Curr Heart Fail Rep 14, 331–337 (2017). https://doi.org/10.1007/s11897-017-0345-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-017-0345-9