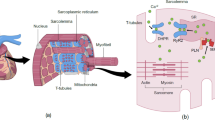
Abstract
Heart failure with preserved ejection fraction (HFpEF) is highly prevalent and is frequently associated with metabolic risk factors. Patients with HFpEF have only a slightly lower mortality than patients with HF and reduced EF. The pathophysiology of HFpEF is currently incompletely understood, which precludes specific therapy. Both HF phenotypes demonstrate distinct cardiac remodeling processes at the macroscopic, microscopic, and ultrastructural levels. Increased diastolic left-ventricular (LV) stiffness and impaired LV relaxation are important features of HFpEF, which can be explained by changes in the extracellular matrix and the cardiomyocytes. In HFpEF, elevated intrinsic cardiomyocyte stiffness contributes to high diastolic LV stiffness. Posttranslational changes in the sarcomeric protein titin, affecting titin isoform expression and phosphorylation, contribute to elevated cardiomyocyte stiffness. Increased nitrosative/oxidative stress, impaired nitric oxide bioavailability, and down-regulation of myocardial cyclic guanosine monophosphate and protein kinase G signaling could trigger posttranslational modifications of titin, thereby augmenting cardiomyocyte and LV diastolic stiffness.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9.
The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). Eur Heart J 2011, Aug 6, epub ahead of print.
Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol. 2010;55:526–37.
• Schwartzenberg S, Redfield MM, From AM, et al. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–51. This article provides evidence that HFpEF and HFrEF represent distinct heart failure phenotypes as hemodynamic responses to acute vasodilator therapy substantially differ between HFpEF and HFrEF patients.
van Heerebeek L, Borbely A, Niessen HWM, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–73.
Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation. 2006;113:296–304.
Yancy CW, Lopatin M, Stevenson LW, et al. For the ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function. A Report from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2006;47:76–84.
Fonorow GC, Stough WG, Abraham WT, et al. OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–77.
Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50.
Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure – abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9.
Westermann D, Kasner M, Steendijk P, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–60.
Borlaug BA, Nishimura RA, Sorajja P, et al. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–95.
Paulus WJ. Culprit Mechanism(s) for Exercise Intolerance in Heart Failure With Normal Ejection Fraction. J Am Coll Cardiol. 2010;56:864–6.
Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis and treatment. Eur Heart J. 2011;32:670–9.
Weber KT, Sun Y, Tyagi SC, Cleutjens JP. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26:279–92.
Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–75.
Martos R, Baugh J, Ledwidge M, et al. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–95.
• Kasner M, Westermann D, Lopez B, et al. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57:977–85. This study demonstrates that extracellular matrix remodelling correlates with diastolic LV dysfunction and reduced exercise capacity in patients with HFpEF.
Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67.
Krum H, Elsik M, Schneider HG, et al. Relation of peripheral collagen markers to death and hospitalization in patients with heart failure and preserved ejection fraction: results of the I-PRESERVE collagen substudy. Circ Heart Fail. 2011;4:561–8.
Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–342.
Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–96.
Martos R, Baugh J, Ledwidge M, et al. Diagnosis of heart failure with preserved ejection fraction: improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail. 2009;11:191–7.
Lindsay MM, Maxwell P, Dunn FG. TIMP-1: a marker of left ventricular diastolic dysfunction and fibrosis in hypertension. Hypertension. 2002;40:136–41.
González A, López B, Querejeta R, et al. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 2010;55:1418–24.
Spinale FG, Coker ML, Heung LJ, et al. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102:1944–9.
• Westermann D, Lindner D, Kasner M, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. This study demonstrates that cardiac inflammation contributes to maladaptive cardiac extracellular matrix remodelling and diastolic LV dysfunction in HFpEF.
Collier P, Watson CJ, Voon V, et al. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087–95.
Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Health ABC Study Investigators. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–37.
Borbely A, van der Velden J, Papp Z, et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–81.
van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: Importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51.
•• Borbely A, Falcao-Pires I, van Heerebeek L, et al. Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–6. This study is of major importance as it links increased cardiomyocyte stiffness to posttranslational modifications of the giant elastic sarcomeric protein titin. A specific titin N2B phosphorylation deficit was demonstrated in heart failure patients which was associated with increased cardiomyocyte stiffness.
• Krüger M, Linke WA. Titin-based mechanical signaling in normal and failing myocardium. J Mol Cell Cardiol. 2009;46:490–8. This review article represents a thorough overview of the important role of titin in determination of cardiomyocyte stiffness and biomechanical signaling.
Chung CS, Granzier HL. Contribution of titin and extracellular matrix to passive pressure and measurement of sarcomere length in the mouse left ventricle. J Mol Cell Cardiol. 2011;50:731–9.
Makarenko I, Opitz CA, Leake MC, et al. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–16.
Nagueh SF, Shah G, Wu Y, et al. Altered titin expression, myocardial stiffness and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–62.
Neagoe C, Kulke M, del Monte F, et al. Titin isoform switch in ischemic human heart disease. Circulation. 2002;106:1333–41.
Krüger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil. 2006;27:435–44.
•• Krüger M, Kötter S, Grützner A, et al. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. This article was the first to demonstrate specific PKG binding sites within the stiff N2B segment of titin and that PKG-mediated phosphorylation of the stiff N2B segment lowers cardiomyocyte stiffness.
Hidalgo C, Hudson B, Bogomolovas J, et al. PKC phosphorylation of titin’s PEVK element. A novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105:631–8.
Grützner A, Garcia-Manyes S, Kötter S, et al. Modulation of titin-based stiffness by disulfide bonding in the cardiac titin N2-B unique sequence. Biophys J. 2009;97:825–34.
• Tsai E, Kass DA. Cyclic GMP signalling in cardiovascular pathology and therapeutics. Pharmacol Ther. 2009;122:216–38. This review article provides comprehensive insight into the importance of cGMP-PKG signaling for cardiovascular physiology and pathophysiology.
Takimoto E, Champion HC, Li M, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–22.
•• Bishu K, Hamdani N, Mohammed SF, et al. Sildenafil and BNP acutely phosphorylate titin and improve diastolic distensbility in vivo. Circulation. 2011;124:2882–91. In an old, hypertensive dog model, enhanced signaling through the cGMP-PKG pathway by either stimulating natriuretic peptide-mediated activation of cGMP-PKG signaling as well as by sildenafil-mediated prevention of cGMP breakdown was shown to increase phosphorylation of titin, which improved diastolic LV distensibility.
• Guazzi M, Vicenzi M, Arena R, et al. PDE-5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry and clinical status in patients with stable systolic heart failure: results of a 1-year prospective, randomized, placebo controlled trial. Circ Heart Fail. 2011;4:8–17. This study demonstrates that sildenafil improves diastolic function, cardiac remodelling and clinical status in HFrEF patients.
•• Guazzi M, Vicenzi M, Arena R, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–74. This study is the first to demonstrate improvements of diastolic function in HFpEF patients with pulmonary hypertension following sildenafil treatment.
•• van Heerebeek L, Hamdani N, Falcao-Pires I, et al.: Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012, In press. This study demonstrated reduced myocardial cGMP concentration and PKG activity in HFpEF compared to HFrEF and aortic stenosis patients. The lower myocardial cGMP concentration and reduced PKG acitivity in HFpEF were related to lower myocardial NO bioavailability due to increased nitrosative/oxidative stress.
Münzel T, Daiber A, Ullrich V, et al. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:1551–7.
Münzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–9.
Paulus WJ, Bronzwaer JGF. Nitric oxide’s role in the heart: control of beating or breathing? Am J Physiol Heart Circ Physiol. 2004;287:H8–H13.
Seddon M, Shah AM, Casadei B. Cardiomyocytes as effectors of nitric oxide signaling. Cardiovasc Res. 2007;75:315–26.
• Hammond J, Balligand JL. Nitric oxide synthase and cyclic GMP signaling in cardiac myocytes: from contractility to remodeling. J Mol Cell Cardiol. 2012;52:330–40. This review provides comprehensive insight into the importance of NO and cGMP signaling in cardiomyocytes.
Paulus WJ, Vantrimpont PJ, Shah AM. Acute effects of nitric oxide on left ventricular relaxation and diastolic distensibility in humans. Assessment by bicoronary sodium nitroprusside infusion. Circulation. 1994;89:2070–8.
Heymes C, Vanderheyden M, Bronzwaer JG, et al. Endomyocardial nitric oxide synthase and left ventricular preload reserve in dilated cardiomyopathy. Circulation. 1999;99:3009–16.
Matter CM, Mandinov L, Kaufmann PA, et al. Effect of NO donors on LV diastolic function in patients with severe pressure-overload hypertrophy. Circulation. 1999;99:2396–401.
Takimoto E, Belardi D, Tocchetti CG, et al. Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation. 2007;115:2159–67.
Tsai EJ, Liu Y, Koitabaschi N, et al. Pressure-overload-induced subcellular relocalization/oxidation of soluble guanylate cyclase in the heart modulates enzyme stimulation. Circ Res. 2012;110:295–303.
Kim HN, Januzzi Jr JL. Natriuretic peptide testing in heart failure. Circulation. 2011;123:2015–9.
Ritchie RH, Irvine JC, Rosenkranz AC, et al. Exploiting cGMP-based therapies for the prevention of left ventricular hypertrophy: NO* and beyond. Pharmacol Ther. 2009;124:279–300.
Iwanaga Y, Nishi I, Furuichi S, et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–8.
Shuai XX, Chen YY, Lu YX, et al. Diagnosis of heart failure with preserved ejection fraction: which parameters and diagnostic strategies are more valuable? Eur J Heart Fail. 2011;13:737–41.
O’Connor CM, Starling RC, Hernandez AF, et al. Effect of Nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43.
• Castro LRV, Schittl J, Fischmeister R. Feedback control through cGMP-dependent protein kinase contributes to differential regulation and compartmentation of cGMP in rat cardiac myocytes. Circ Res. 2010;107:1232–40. This study demonstrates that cGMP-PKG signaling is subject to complex differential spatiotemporal regulation and distinct compartmentation with the existence of specific cGMP-PKG “signalosomes” for either natriuretic peptide or NO.
Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res. 2007;100:1569–78.
Mongillo M, Tocchetti CG, Terrin A, et al. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res. 2006;98:226–34.
Lee DI, Vahebi S, Tocchetti CG, et al. PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol. 2010;105:337–47.
Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6.
Solomon SD, Janardhanan R, Verma A, et al. For the Valsartan In Diastolic Dysfunction (VALIDD) Investigators. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–87.
Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008;77:637–48.
• Krüger M, Linke WA. The giant protein titin: a regulatory node that integrates myocyte signaling pathways. J Biol Chem. 2011;286:9905–12. This review article shows the importance of titin in biomechanical signaling and regulation of cardiac remodelling.
Trochu JN, Bouhour JB, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism: implications in health and disease. Circ Res. 2000;87:1108–17.
Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–7.
Smith CS, Bottomley PA, Schulman SP, et al. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation. 2006;114:1151–8.
Diamant M, Lamb HJ, Groeneveld Y, et al. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42:328–35.
Rider OJ, Francis JM, Ali MK, et al. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation. 2012;125:1511–9.
Nediani C, Raimondi L, Borchi E, Cerbai E. Nitric oxide/reactive oxygen species generation and nitroso/redox imbalance in heart failure: from molecular mechanisms to therapeutic implications. Antiox Redox Signal. 2011;14:289–331.
Silberman GA, Fan THM, Liu H, et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–28.
Westermann D, Riad A, Richter U, et al. Enhancement of the endothelial NO synthase attenuates experimental diastolic heart failure. Basic Res Cardiol. 2009;104:499–509.
Wilson Tang WH, Tong W, Shrestha K, et al. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur Heart J. 2008;29:2506–13.
McMurray JJV, Carson PE, Komajda M, et al. Heart failure with preserved ejection fraction: Clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail. 2008;10:149–56.
Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome and diabetes. J Am Coll Cardiol. 2010;55:283–93.
Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13.
Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34.
Ingelsson E, Sundström J, Ärnlöv J, et al. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–41.
Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16.
• Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function. A community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–74. HFpEF patients demonstrate a high prevalence of metabolic risk factors and obesity induces diastolic LV dysfunction independent of other cardiovascular risk factors.
Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–5.
• Dinh W, Lankisch M, Nickl W, et al. Insulin resistance and glycemic abnormalities are associated with deterioration of left ventricular diastolic function: a cross-sectional study. Cardiovasc Diabet. 2010;9:63–76. This study demonstrates that insulin resistance and glycemic abnormalities induce diastolic LV dysfunction.
De las Fuentes L, Brown AL, Mathews SJ, et al. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of LV mass. Eur Heart J. 2007;28:553–9.
Falcao-Pires I, Hamdani N, Borbely A, et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124:1151–9.
Abel ED, Litwin ES, Sweeney G. Cardiac remodelling in obesity. Physiol Rev. 2008;88:389–419.
Falcao-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2011;17:325–44.
Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy. J Am Coll Cardiol. 2008;51:93–102.
Liu S, Ma X, Gong M, et al. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic Biol Med. 2007;42:852–63.
Giannetta E, Isidori AM, Galea N, et al. Chronic Inhibition of cGMP Phosphodiesterase 5A Improves Diabetic Cardiomyopathy: A Randomized, Controlled Clinical Trial Using Magnetic Resonance Imaging With Myocardial Tagging. Circulation. 2012;125:2323–33.
• Rizzo NO, Maloney E, Pham M, et al. Reduced NO-cGMP signaling contributes to vascular inflammation and insulin resistance induced by high-fat feeding. Arterioscler Thromb Vasc Biol. 2010;30:758–65. This study links oxidative stress, inflammation and insulin resistance to downregulation of NO-cGMP signaling and vascular dysfunction. Inhibition of cGMP breakdown by sildenafil abrogated the detrimental vascular effects of high fat feeding and prevented vascular inflammation and insulin resistance.
Krüger M, Babicz K, von Frieling-Salewsky M, Linke WA. Insulin signaling regulates cardiac titin properties in heart development and diabetic cardiomyopathy. J Mol Cell Cardiol. 2010;48:910–6.
Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904.
Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–31.
Connelly KA, Kelly DJ, Zhang Y, et al. Inhibition of protein kinase C-beta by ruboxistaurin preserves cardiac function and reduces extracellular matrix production in diabetic cardiomyopathy. Circ Heart Fail. 2009;2:129–37.
Acknowledgments
L. van Heerebeek, C.P.M. Franssen, N. Hamdani, and W.J. Paulus are funded through a grant from European Commission FP7 Health 2010, Large Collaborative Research Project on Diastolic Heart Failure MEDIA (261409).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Heerebeek, L., Franssen, C.P.M., Hamdani, N. et al. Molecular and Cellular Basis for Diastolic Dysfunction. Curr Heart Fail Rep 9, 293–302 (2012). https://doi.org/10.1007/s11897-012-0109-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-012-0109-5
Keywords
- Cardiomyocytes
- Cyclic guanosine monophosphate
- Diabetes mellitus
- Diastole
- Diastolic dysfunction
- Extracellular matrix
- Fibrosis
- Guanylate cyclase
- Heart failure
- Hypertrophy
- Inflammation
- Metabolic risk factors
- Metabolic syndrome
- Nitric oxide
- Natriuretic peptides
- Obesity
- Oxidative stress
- Protein Kinase G
- Phosphodiesterase
- Titin