Abstract
Purpose of Review
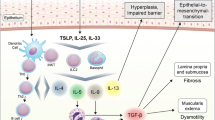
Compelling evidence over the past decade supports the central role of epithelial barrier dysfunction in the pathophysiology of eosinophilic esophagitis (EoE). The purpose of this review is to summarize the genetic, environmental, and immunologic factors driving epithelial barrier dysfunction, and how this impaired barrier can further promote the inflammatory response in EoE.
Recent Findings
Common environmental exposures, such as detergents, may have a direct impact on the esophageal epithelial barrier. In addition, the effects of IL-13 on barrier dysfunction may be reduced by 17β-estradiol, Vitamin D, and the short chain fatty acids butyrate and propionate, suggesting novel therapeutic targets.
Summary
There are many genetic, environmental, and immunologic factors that contribute to epithelial barrier dysfunction in EoE. This leads to further skewing of the immune response to a “Th2” phenotype, alterations in the esophageal microbiome, and penetration of relevant antigens into the esophageal mucosa, which are central to the pathophysiology of EoE.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology. 1977;72(6):1312–6.
Kerlin P, Jones D, Remedios M, Campbell C. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol. 2007;41(4):356–61. https://doi.org/10.1097/01.mcg.0000225590.08825.77.
Kirchner GI, Zuber-Jerger I, Endlicher E, Gelbmann C, Ott C, Ruemmele P, et al. Causes of bolus impaction in the esophagus. Surg Endosc. 2011;25(10):3170–4. https://doi.org/10.1007/s00464-011-1681-6.
Diniz LO, Towbin AJ. Causes of esophageal food bolus impaction in the pediatric population. Dig Dis Sci. 2012;57(3):690–3. https://doi.org/10.1007/s10620-011-1911-8.
Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155(4):1022-33.e10. https://doi.org/10.1053/j.gastro.2018.07.009.
Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109(5):1503–12. https://doi.org/10.1016/0016-5085(95)90637-1.
Peterson KA, Byrne KR, Vinson LA, Ying J, Boynton KK, Fang JC, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol. 2013;108(5):759–66. https://doi.org/10.1038/ajg.2012.468.
Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–102. https://doi.org/10.1016/j.cgh.2006.05.026.
Molina-Infante J, Arias Á, Alcedo J, Garcia-Romero R, Casabona-Frances S, Prieto-Garcia A, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2–4-6 study. J Allergy Clin Immunol. 2018;141(4):1365–72. https://doi.org/10.1016/j.jaci.2017.08.038.
Cookson WO, Ubhi B, Lawrence R, Abecasis GR, Walley AJ, Cox HE, et al. Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nat Genet. 2001;27(4):372–3. https://doi.org/10.1038/86867.
Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–6. https://doi.org/10.1038/ng1767.
Irvine AD. Fleshing out filaggrin phenotypes. J Invest Dermatol. 2007;127(3):504–7. https://doi.org/10.1038/sj.jid.5700695.
Blanchard C, Stucke EM, Burwinkel K, Caldwell JM, Collins MH, Ahrens A, et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010;184(7):4033–41. https://doi.org/10.4049/jimmunol.0903069.
Katzka DA, Tadi R, Smyrk TC, Katarya E, Sharma A, Geno DM, et al. Effects of topical steroids on tight junction proteins and spongiosis in esophageal epithelia of patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12(11):1824-9.e1. https://doi.org/10.1016/j.cgh.2014.02.039.
Katzka DA, Ravi K, Geno DM, Smyrk TC, Iyer PG, Alexander JA, et al. Endoscopic mucosal impedance measurements correlate with eosinophilia and dilation of intercellular spaces in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13(7):1242-8.e1. https://doi.org/10.1016/j.cgh.2014.12.032.
Vaezi MF, Choksi Y. Mucosal impedance: a new way to diagnose reflux disease and how it could change your practice. Am J Gastroenterol. 2017;112(1):4–7. https://doi.org/10.1038/ajg.2016.513.
Patel DA, Higginbotham T, Slaughter JC, Aslam M, Yuksel E, Katzka D, et al. Development and validation of a mucosal impedance contour analysis system to distinguish esophageal disorders. Gastroenterology. 2019;156(6):1617-26.e1. https://doi.org/10.1053/j.gastro.2019.01.253.
Sifrim D, Roman S, Savarino E, Bor S, Bredenoord AJ, Castell D, et al. Normal values and regional differences in oesophageal impedance-pH metrics: a consensus analysis of impedance-pH studies from around the world. Gut. 2020. https://doi.org/10.1136/gutjnl-2020-322627.
Wallace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology. 2009;136(5):1509–13. https://doi.org/10.1053/j.gastro.2009.03.034.
Chu CL, Zhen YB, Lv GP, Li CQ, Li Z, Qi QQ, et al. Microalterations of esophagus in patients with non-erosive reflux disease: in-vivo diagnosis by confocal laser endomicroscopy and its relationship with gastroesophageal reflux. Am J Gastroenterol. 2012;107(6):864–74. https://doi.org/10.1038/ajg.2012.44.
Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4(8):979–87. https://doi.org/10.1016/j.cgh.2006.05.010.
Fritscher-Ravens A, Schuppan D, Ellrichmann M, Schoch S, Röcken C, Brasch J, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2014;147(5):1012-20.e4. https://doi.org/10.1053/j.gastro.2014.07.046.
Blevins CH, Sharma AN, Johnson ML, Geno D, Gupta M, Bharucha AE, et al. Influence of reflux and central obesity on intercellular space diameter of esophageal squamous epithelium. U Eur Gastroenterol J. 2016;4(2):177–83. https://doi.org/10.1177/2050640615598426.
van Rhijn BD, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RM, de Jonge WJ, et al. Histological response to fluticasone propionate in patients with eosinophilic esophagitis is associated with improved functional esophageal mucosal integrity. Am J Gastroenterol. 2015;110(9):1289–97. https://doi.org/10.1038/ajg.2015.247.
Wu L, Oshima T, Li M, Tomita T, Fukui H, Watari J, et al. Filaggrin and tight junction proteins are crucial for IL-13-mediated esophageal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2018;315(3):G341–50. https://doi.org/10.1152/ajpgi.00404.2017.
Simon D, Page B, Vogel M, Bussmann C, Blanchard C, Straumann A, et al. Evidence of an abnormal epithelial barrier in active, untreated and corticosteroid-treated eosinophilic esophagitis. Allergy. 2018;73(1):239–47. https://doi.org/10.1111/all.13244.
Masterson JC, Biette KA, Hammer JA, Nguyen N, Capocelli KE, Saeedi BJ, et al. Epithelial HIF-1α/claudin-1 axis regulates barrier dysfunction in eosinophilic esophagitis. J Clin Invest. 2019;129(8):3224–35. https://doi.org/10.1172/jci126744.
Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014;7(3):718–29. https://doi.org/10.1038/mi.2013.90.
de Rooij WE, Diks MAP, Warners MJ, Ampting M, van Esch B, Bredenoord AJ. Gene expression and clinical outcomes after dietary treatment for eosinophilic esophagitis: a prospective study. Neurogastroenterol Motil. 2022;34(10):e14367. https://doi.org/10.1111/nmo.14367.
van Rhijn BD, Weijenborg PW, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RM, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12(11):1815-23.e2. https://doi.org/10.1016/j.cgh.2014.02.037.
Lowry MA, Vaezi MF, Correa H, Higginbotham T, Slaughter JC, Acra S. Mucosal impedance measurements differentiate pediatric patients with active versus inactive eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2018;67(2):198–203. https://doi.org/10.1097/mpg.0000000000001943.
Alexander JA, Ravi K, Geno DM, Tholen CJ, Higginbotham TC, Wildhorn S, et al. Comparison of mucosal impedance measurements throughout the esophagus and mucosal eosinophil counts in endoscopic biopsy specimens in eosinophilic esophagitis. Gastrointest Endosc. 2019;89(4):693-700.e1. https://doi.org/10.1016/j.gie.2018.08.031.
Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42(4):289–91. https://doi.org/10.1038/ng.547.
Kottyan LC, Davis BP, Sherrill JD, Liu K, Rochman M, Kaufman K, et al. Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat Genet. 2014;46(8):895–900. https://doi.org/10.1038/ng.3033.
Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116(2):536–47. https://doi.org/10.1172/jci26679.
Kottyan LC, Rothenberg ME. Genetics of eosinophilic esophagitis. Mucosal Immunol. 2017;10(3):580–8. https://doi.org/10.1038/mi.2017.4.
Kottyan LC, Parameswaran S, Weirauch MT, Rothenberg ME, Martin LJ. The genetic etiology of eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145(1):9–15. https://doi.org/10.1016/j.jaci.2019.11.013.
Kalb B, Marenholz I, Jeanrenaud A, Meixner L, Arnau-Soler A, Rosillo-Salazar OD, et al. Filaggrin loss-of-function mutations are associated with persistence of egg and milk allergy. J Allergy Clin Immunol. 2022;150(5):1125–34. https://doi.org/10.1016/j.jaci.2022.05.018. This study demonstrates that FLG loss-of-function mutations are a risk factor for food allergy independent of eczema.
Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127(3):661–7. https://doi.org/10.1016/j.jaci.2011.01.031.
Rice NE, Patel BD, Lang IA, Kumari M, Frayling TM, Murray A, et al. Filaggrin gene mutations are associated with asthma and eczema in later life. J Allergy Clin Immunol. 2008;122(4):834–6. https://doi.org/10.1016/j.jaci.2008.07.027.
Matoso A, Mukkada VA, Lu S, Monahan R, Cleveland K, Noble L, et al. Expression microarray analysis identifies novel epithelial-derived protein markers in eosinophilic esophagitis. Mod Pathol. 2013;26(5):665–76. https://doi.org/10.1038/modpathol.2013.41.
Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120(6):1292–300. https://doi.org/10.1016/j.jaci.2007.10.024.
Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19(8):1005–13. https://doi.org/10.1038/nm.3281.
Sherrill JD, Gao PS, Stucke EM, Blanchard C, Collins MH, Putnam PE, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126(1):160-5.e3. https://doi.org/10.1016/j.jaci.2010.04.037.
Hammad H, Lambrecht BN. Barrier epithelial cells and the control of Type 2 immunity. Immunity. 2015;43(1):29–40. https://doi.org/10.1016/j.immuni.2015.07.007.
Chandramouleeswaran PM, Shen D, Lee AJ, Benitez A, Dods K, Gambanga F, et al. Preferential secretion of Thymic stromal Lymphopoietin (TSLP) by terminally differentiated esophageal epithelial cells: relevance to eosinophilic esophagitis (EoE). PLoS ONE. 2016;11(3):e0150968. https://doi.org/10.1371/journal.pone.0150968.
Ramasamy A, Kuokkanen M, Vedantam S, Gajdos ZK, Couto Alves A, Lyon HN, et al. Genome-wide association studies of asthma in population-based cohorts confirm known and suggested loci and identify an additional association near HLA. PLoS One. 2012;7(9):e44008. https://doi.org/10.1371/journal.pone.0044008.
Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–21. https://doi.org/10.1056/NEJMoa0906312.
Martin LJ, He H, Collins MH, Abonia JP, Biagini Myers JM, Eby M, et al. Eosinophilic esophagitis (EoE) genetic susceptibility is mediated by synergistic interactions between EoE-specific and general atopic disease loci. J Allergy Clin Immunol. 2018;141(5):1690–8. https://doi.org/10.1016/j.jaci.2017.09.046.
Judd LM, Heine RG, Menheniott TR, Buzzelli J, O’Brien-Simpson N, Pavlic D, et al. Elevated IL-33 expression is associated with pediatric eosinophilic esophagitis, and exogenous IL-33 promotes eosinophilic esophagitis development in mice. Am J Physiol Gastrointest Liver Physiol. 2016;310(1):G13-25. https://doi.org/10.1152/ajpgi.00290.2015.
Travers J, Rochman M, Caldwell JM, Besse JA, Miracle CE, Rothenberg ME. IL-33 is induced in undifferentiated, non-dividing esophageal epithelial cells in eosinophilic esophagitis. Sci Rep. 2017;7(1):17563. https://doi.org/10.1038/s41598-017-17541-5.
Marwaha AK, Laxer R, Liang M, Muise AM, Eiwegger T. A chromosomal duplication encompassing Interleukin-33 causes a novel hyper IgE phenotype characterized by eosinophilic esophagitis and generalized autoimmunity. Gastroenterology. 2022;163(2):510-3.e3. https://doi.org/10.1053/j.gastro.2022.04.026.
Sleiman PM, Wang ML, Cianferoni A, Aceves S, Gonsalves N, Nadeau K, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. 2014;5:5593. https://doi.org/10.1038/ncomms6593.
Davis BP, Stucke EM, Khorki ME, Litosh VA, Rymer JK, Rochman M, et al. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. 2016;1(4):e86355. https://doi.org/10.1172/jci.insight.86355.
Litosh VA, Rochman M, Rymer JK, Porollo A, Kottyan LC, Rothenberg ME. Calpain-14 and its association with eosinophilic esophagitis. J Allergy Clin Immunol. 2017;139(6):1762-71.e7. https://doi.org/10.1016/j.jaci.2016.09.027.
Alexander ES, Martin LJ, Collins MH, Kottyan LC, Sucharew H, He H, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol. 2014;134(5):1084-92.e1. https://doi.org/10.1016/j.jaci.2014.07.021.
Jensen ET, Kuhl JT, Martin LJ, Rothenberg ME, Dellon ES. Prenatal, intrapartum, and postnatal factors are associated with pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2018;141(1):214–22. https://doi.org/10.1016/j.jaci.2017.05.018.
Kurt G, Svane HML, Erichsen R, Heide-Jørgensen U, Sørensen HT, Dellon ES, et al. Prenatal, intrapartum, and neonatal factors increase the risk of eosinophilic esophagitis. Am J Gastroenterol. 2023. https://doi.org/10.14309/ajg.0000000000002303. This study identified environmental factors, including prenatal, intrapartum, and neonatal factors, associated with the development of EoE.
Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology. 2018;154(2):319-32.e3. https://doi.org/10.1053/j.gastro.2017.06.067.
Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739–51. https://doi.org/10.1038/s41577-021-00538-7.
Singer MM, Tjeerdema RS. Fate and effects of the surfactant sodium dodecyl sulfate. Rev Environ Contam Toxicol. 1993;133:95–149. https://doi.org/10.1007/978-1-4613-9529-4_3.
Xian M, Wawrzyniak P, Rückert B, Duan S, Meng Y, Sokolowska M, et al. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J Allergy Clin Immunol. 2016;138(3):890-3.e9. https://doi.org/10.1016/j.jaci.2016.07.003.
Wang M, Tan G, Eljaszewicz A, Meng Y, Wawrzyniak P, Acharya S, et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol. 2019;143(5):1892–903. https://doi.org/10.1016/j.jaci.2018.11.016.
Doyle AD, Masuda MY, Pyon GC, Luo H, Putikova A, LeSuer WE, et al. Detergent exposure induces epithelial barrier dysfunction and eosinophilic inflammation in the esophagus. Allergy. 2023;78(1):192–201. https://doi.org/10.1111/all.15457. This study assessed the harmful effect of SDS exposure to eosophageal muscosa via in vitro as well as in vivo mouse models and found that detergents may be a key environmental trigger in EoE pathogenesis.
Hosoki K, Boldogh I, Sur S. Innate responses to pollen allergens. Curr Opin Allergy Clin Immunol. 2015;15(1):79–88. https://doi.org/10.1097/aci.0000000000000136.
Vinhas R, Cortes L, Cardoso I, Mendes VM, Manadas B, Todo-Bom A, et al. Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides. Allergy. 2011;66(8):1088–98. https://doi.org/10.1111/j.1398-9995.2011.02598.x.
Wan H, Winton HL, Soeller C, Taylor GW, Gruenert DC, Thompson PJ, et al. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2001;31(2):279–94. https://doi.org/10.1046/j.1365-2222.2001.00970.x.
Ravi A, Marietta EV, Geno DM, Alexander JA, Murray JA, Katzka DA. Penetration of the esophageal epithelium by dust mite antigen in patients with eosinophilic esophagitis. Gastroenterology. 2019;157(1):255–6. https://doi.org/10.1053/j.gastro.2019.02.042.
Armentia A, Martín-Armentia S, Álvarez-Nogal R, Armentia BM, Gayoso MJ, Fernández-González D. Germination of pollen grains in the oesophagus of individuals with eosinophilic oesophagitis. Clin Exp Allergy. 2019;49(4):471–3. https://doi.org/10.1111/cea.13312.
Caraballo JC, Yshii C, Westphal W, Moninger T, Comellas AP. Ambient particulate matter affects occludin distribution and increases alveolar transepithelial electrical conductance. Respirology. 2011;16(2):340–9. https://doi.org/10.1111/j.1440-1843.2010.01910.x.
Zhao R, Guo Z, Zhang R, Deng C, Xu J, Dong W, et al. Nasal epithelial barrier disruption by particulate matter ≤2.5 μm via tight junction protein degradation. J Appl Toxicol. 2018;38(5):678–87. https://doi.org/10.1002/jat.3573.
Bleck B, Grunig G, Chiu A, Liu M, Gordon T, Kazeros A, et al. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J Immunol. 2013;190(7):3757–63. https://doi.org/10.4049/jimmunol.1201165.
Muir AB, Wang JX, Nakagawa H. Epithelial-stromal crosstalk and fibrosis in eosinophilic esophagitis. J Gastroenterol. 2019;54(1):10–8. https://doi.org/10.1007/s00535-018-1498-3.
Gann PH, Deaton RJ, McMahon N, Collins MH, Dellon ES, Hirano I, et al. An anti-IL-13 antibody reverses epithelial-mesenchymal transition biomarkers in eosinophilic esophagitis: Phase 2 trial results. J Allergy Clin Immunol. 2020;146(2):367-76.e3. https://doi.org/10.1016/j.jaci.2020.03.045. This phase 2 clinical trial demonstrates that pharmacologic inhibition of IL-13 (anti‒IL-13 mAb) could be a potential therapy for fibrostenosis, the most serious eosinophilic esophagitis (EoE) complication.
Wheeler JC, Vanoni S, Zeng C, Waggoner L, Yang Y, Wu D, et al. 17β-Estradiol protects the esophageal epithelium from IL-13-induced barrier dysfunction and remodeling. J Allergy Clin Immunol. 2019;143(6):2131–46. https://doi.org/10.1016/j.jaci.2018.10.070.
Brusilovsky M, Rochman M, Shoda T, Kotliar M, Caldwell JM, Mack LE, et al. Vitamin D receptor and STAT6 interactome governs oesophageal epithelial barrier responses to IL-13 signalling. Gut. 2023;72(5):834–45. https://doi.org/10.1136/gutjnl-2022-327276.
Kleuskens MTA, Haasnoot ML, Herpers BM, Ampting M, Bredenoord AJ, Garssen J, et al. Butyrate and propionate restore interleukin 13-compromised esophageal epithelial barrier function. Allergy. 2022;77(5):1510–21. https://doi.org/10.1111/all.15069.
Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119(1):206–12. https://doi.org/10.1016/j.jaci.2006.10.016.
Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-β1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126(6):1198-204.e4. https://doi.org/10.1016/j.jaci.2010.08.050.
Nguyen N, Fernando SD, Biette KA, Hammer JA, Capocelli KE, Kitzenberg DA, et al. TGF-β1 alters esophageal epithelial barrier function by attenuation of claudin-7 in eosinophilic esophagitis. Mucosal Immunol. 2018;11(2):415–26. https://doi.org/10.1038/mi.2017.72.
Laky K, Kinard JL, Li JM, Moore IN, Lack J, Fischer ER, et al. Epithelial-intrinsic defects in TGFβR signaling drive local allergic inflammation manifesting as eosinophilic esophagitis. Sci Immunol. 2023;8(79):eabp9940. https://doi.org/10.1126/sciimmunol.abp9940.
Salamon P, Shoham NG, Puxeddu I, Paitan Y, Levi-Schaffer F, Mekori YA. Human mast cells release oncostatin M on contact with activated T cells: possible biologic relevance. J Allergy Clin Immunol. 2008;121(2):448-55.e5. https://doi.org/10.1016/j.jaci.2007.08.054.
Suda T, Chida K, Todate A, Ide K, Asada K, Nakamura Y, et al. Oncostatin M production by human dendritic cells in response to bacterial products. Cytokine. 2002;17(6):335–40. https://doi.org/10.1006/cyto.2002.1023.
Tamura S, Morikawa Y, Miyajima A, Senba E. Expression of oncostatin M in hematopoietic organs. Dev Dyn. 2002;225(3):327–31. https://doi.org/10.1002/dvdy.10156.
Pothoven KL, Norton JE, Hulse KE, Suh LA, Carter RG, Rocci E, et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J Allergy Clin Immunol. 2015;136(3):737-46.e4. https://doi.org/10.1016/j.jaci.2015.01.043.
Kleuskens MTA, Bek MK, Al Halabi Y, Blokhuis BRJ, Diks MAP, Haasnoot ML, et al. Mast cells disrupt the function of the esophageal epithelial barrier. Mucosal Immunol. 2023. https://doi.org/10.1016/j.mucimm.2023.06.001.
Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59(1):21–30. https://doi.org/10.1136/gut.2009.178558.
Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593–604. https://doi.org/10.1053/j.gastro.2011.07.044.
Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G 3rd, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):456–63. https://doi.org/10.1016/j.jaci.2011.11.044. (63.e1-3).
Benitez AJ, Hoffmann C, Muir AB, Dods KK, Spergel JM, Bushman FD, et al. Inflammation-associated microbiota in pediatric eosinophilic esophagitis. Microbiome. 2015;3:23. https://doi.org/10.1186/s40168-015-0085-6.
Harris JK, Fang R, Wagner BD, Choe HN, Kelly CJ, Schroeder S, et al. Esophageal microbiome in eosinophilic esophagitis. PLoS ONE. 2015;10(5):e0128346. https://doi.org/10.1371/journal.pone.0128346.
Norder Grusell E, Dahlén G, Ruth M, Bergquist H, Bove M. The cultivable bacterial flora of the esophagus in subjects with esophagitis. Scand J Gastroenterol. 2018;53(6):650–6. https://doi.org/10.1080/00365521.2018.1457712.
Massimino L, Barchi A, Mandarino FV, Spanò S, Lamparelli LA, Vespa E, et al. A multi-omic analysis reveals the esophageal dysbiosis as the predominant trait of eosinophilic esophagitis. J Transl Med. 2023;21(1):46. https://doi.org/10.1186/s12967-023-03898-x. This study employed advanced bioinformatics and reanalyzed RNA-seq data of 18 different studies on EoE. This approach identified new EoE-specific molecular markers and also revealed microbiota dysbiosis as a predominant characteristic of EoE pathogenesis.
Ravi A, Marietta EV, Alexander JA, Murray JA, Katzka DA (2023) H influenza LPS colocalization with Toll-like receptor 4 in eosinophilic esophagitis. JACI Global 2(4):100151
Ravi A, Marietta EV, Alexander JA, Peterson K, Lavey C, Geno DM, et al. Mucosal penetration and clearance of gluten and milk antigens in eosinophilic oesophagitis. Aliment Pharmacol Ther. 2021;53(3):410–7. https://doi.org/10.1111/apt.16180.
Medernach JG, Li RC, Zhao XY, Yin B, Noonan EA, Etter EF, et al. Immunoglobulin G4 in eosinophilic esophagitis: Immune complex formation and correlation with disease activity. Allergy. 2023. https://doi.org/10.1111/all.15826. These data demonstrate that IgG4 and major cow's milk proteins are forming immune complexes in the esophageal tissue of patients with active EoE.
Funding
This work was funded by the NIH through the following grants: 1R01AI175232 (ECM).
Author information
Authors and Affiliations
Contributions
ECM, RS and DK served as the primary authors. RS prepared figures 1 and 2. All authors reviewed the manuscript and approved the final draft submitted.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Potential Competing Interests
ECM receives grant support from the NIH/NIAID and consulting fees from Regeneron. DAK received an honorarium from Sanofi and Medtronic.
Human and Animal Rights and Informed Consent
This article does not present any novel data on studies with human or animal subjects performed by any of the authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McGowan, E.C., Singh, R. & Katzka, D.A. Barrier Dysfunction in Eosinophilic Esophagitis. Curr Gastroenterol Rep 25, 380–389 (2023). https://doi.org/10.1007/s11894-023-00904-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-023-00904-6