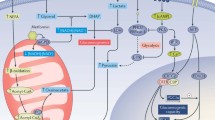
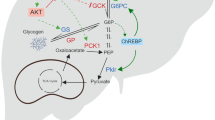
Abstract
Purpose of Review
The purpose of this review is to provide a brief summary of recent advances in our understanding of liver metabolism. The critical role of the liver in controlling whole-body energy homeostasis makes such understanding crucial to efficiently design new treatments for metabolic syndrome diseases, including type 2 diabetes (T2D).
Recent Findings
Significant advances have been made regarding our understanding of the direct and indirect effects of insulin on hepatic metabolism and the communication between the liver and other tissues. Moreover, the catabolic functions of glucagon, as well as the importance of hepatic redox status for the regulation of glucose production, are emerging as potential targets to reduce hyperglycemia.
Summary
A resolution to the long-standing question “insulin suppression of hepatic glucose production, direct or indirect effect?” is starting to emerge. New advances in our understanding of important fasting-induced hepatic metabolic fluxes may help design better therapies for T2D.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6–12. https://doi.org/10.1089/pop.2015.0181.
Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–223. https://doi.org/10.1152/physrev.00063.2017.
Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13(10):572–87. https://doi.org/10.1038/nrendo.2017.80.
Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14(1):9–19. https://doi.org/10.1016/j.cmet.2011.06.003.
Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr. 2012;3(3):286–94. https://doi.org/10.3945/an.112.002089.
Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284(4):E671–8. https://doi.org/10.1152/ajpendo.00492.2002.
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. https://doi.org/10.1038/414799a.
Sharabi K, Tavares CD, Rines AK, Puigserver P. Molecular pathophysiology of hepatic glucose production. Mol Asp Med. 2015;46:21–33. https://doi.org/10.1016/j.mam.2015.09.003.
Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152(4):673–84. https://doi.org/10.1016/j.cell.2013.01.041.
Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–71. https://doi.org/10.1016/j.cell.2012.02.017.
Titchenell PM, Lazar MA, Birnbaum MJ. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol Metab. 2017;28(7):497–505. https://doi.org/10.1016/j.tem.2017.03.003.
Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci. 2018;1411(1):21–35. https://doi.org/10.1111/nyas.13435.
Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6(1):87–97.
Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18(3):388–95. https://doi.org/10.1038/nm.2686.
Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, et al. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8(1):65–76. https://doi.org/10.1016/j.cmet.2008.06.006.
O-Sullivan I, Zhang W, Wasserman DH, Liew C, Liu J, Paik J, et al. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat Commun. 2015;6(1). https://doi.org/10.1038/ncomms8079.
• Tao R, Wang C, Stöhr O, Qiu W, Hu Y, Miao J, et al. Inactivating hepatic follistatin alleviates hyperglycemia. Nat Med. 2018;24(7):1058–69. https://doi.org/10.1038/s41591-018-0048-0. Findings from this study identify a potential link between FoxO1 activity in liver and insulin resistance in WAT. These findings help to clarify the relation between the direct and indirect effects of insulin on hepatic glucose production.
Titchenell PM, Chu Q, Monks BR, Birnbaum MJ. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nat Commun. 2015;6(1):7078. https://doi.org/10.1038/ncomms8078.
Titchenell PM, Quinn WJ, Lu M, Chu Q, Lu W, Li C, et al. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metab. 2016;23(6):1154–66. https://doi.org/10.1016/j.cmet.2016.04.022.
Edgerton DS, Kraft G, Smith M, Farmer B, Williams PE, Coate KC, et al. Insulin’s direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight. 2017;2(6):e91863. https://doi.org/10.1172/jci.insight.91863.
Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48(5):1198–214.
Cherrington AD, Moore MC, Sindelar DK, Edgerton DS. Insulin action on the liver in vivo. Biochem Soc Trans. 2007;35(Pt 5):1171–4. https://doi.org/10.1042/BST0351171.
Perry RJ, Camporez J-PGG, Kursawe R, Titchenell PM, Zhang D, Perry CJ, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745–58. https://doi.org/10.1016/j.cell.2015.01.012.
Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6(3):208–16. https://doi.org/10.1016/j.cmet.2007.08.006.
Haeusler RA, Hartil K, et al. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nat Commun. 2014;5:5190.
Zhang W, Patil S, Chauhan B, Guo S, et al. FoxO1 regulates multiple metabolic pathways in the liver effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006. https://doi.org/10.1074/jbc.M600272200.
Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7(2):125–34. https://doi.org/10.1016/j.cmet.2007.11.013.
Haas JT, Miao J, Chanda D, Wang Y, Zhao E, Haas ME, et al. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 2012;15(6):873–84. https://doi.org/10.1016/j.cmet.2012.05.002.
Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–31. https://doi.org/10.1172/JCI15593.
Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, et al. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012;15(2):240–6. https://doi.org/10.1016/j.cmet.2011.12.017.
Langlet F, Haeusler RA, Lindén D, Ericson E, Norris T, Johansson A, et al. Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell. 2017;171(4):824–835.e18. https://doi.org/10.1016/j.cell.2017.09.045.
Sharabi K, Lin H, Tavares CDJ, Dominy JE, Camporez JP, Perry RJ, et al. Selective chemical inhibition of PGC-1alpha gluconeogenic activity ameliorates type 2 diabetes. Cell. 2017;169(1):148–60 e15. https://doi.org/10.1016/j.cell.2017.03.001.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–8. https://doi.org/10.1038/nature03354.
Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3(6):429–38. https://doi.org/10.1016/j.cmet.2006.04.013.
Lee Y, Dominy JE, Choi YJ, Jurczak M, Tolliday N, Camporez JP, et al. Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature. 2014;510(7506):547–51. https://doi.org/10.1038/nature13267.
Dominy JE Jr, Lee Y, Jedrychowski MP, Chim H, Jurczak MJ, Camporez JP, et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol Cell. 2012;48(6):900–13. https://doi.org/10.1016/j.molcel.2012.09.030.
Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4–12. https://doi.org/10.1172/JCI60016.
Longuet C, Robledo AM, Dean ED, Dai C, Ali S, McGuinness I, et al. Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia. Evidence for a Circulating α-Cell Growth Factor. Diabetes. 2013;62(4):1196–205. https://doi.org/10.2337/db11-1605.
Dean DE, Li M, Prasad N, Wisniewski SN, Deylen A, Spaeth J, et al. Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. Cell Metab. 2017;25(6):1362–137300000. https://doi.org/10.1016/j.cmet.2017.05.011.
Kim J, Okamoto H, Huang Z, Anguiano G, Chen S, Liu Q, et al. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. Cell Metab. 2017;25(6):1348–1361.e8. https://doi.org/10.1016/j.cmet.2017.05.006.
Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–37. https://doi.org/10.1016/j.cmet.2013.04.008.
Soni H. Peptide-based GLP-1/glucagon co-agonists: a double-edged sword to combat diabesity. Med Hypotheses. 2016;95:5–9. https://doi.org/10.1016/j.mehy.2016.08.005.
Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5(10):749–57. https://doi.org/10.1038/nchembio.209.
Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58(10):2258–66. https://doi.org/10.2337/db09-0278.
Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschop MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. 2010;6(12):689–97. https://doi.org/10.1038/nrendo.2010.187.
Longuet C, Sinclair EM, Maida A, Baggio LL, Maziarz M, Charron MJ, et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008;8(5):359–71. https://doi.org/10.1016/j.cmet.2008.09.008.
• Miller RA, Shi Y, Lu W, Pirman DA, Jatkar A, Blatnik M, et al. Targeting hepatic glutaminase activity to ameliorate hyperglycemia. Nat Med. 2018;24(4):518. https://doi.org/10.1038/nm.4514. This study rigorously charechterizes changes in hepatic metabolic fluxes in response to glucagon stimulation, and identifies glutaminase as a potential target to control hyperglycemia.
Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5(4):313–20. https://doi.org/10.1016/j.cmet.2007.03.004.
Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2015;125(12):4447–62. https://doi.org/10.1172/JCI82204.
Cappel DA, Deja S, Duarte JAG, Kucejova B, Inigo M, Fletcher JA, et al. Pyruvate-carboxylase-mediated anaplerosis promotes antioxidant capacity by sustaining TCA cycle and redox metabolism in liver. Cell Metab. 2019;29(6):1291–305 e8. https://doi.org/10.1016/j.cmet.2019.03.014.
Madiraju AK, Erion DM, Rahimi Y, Zhang X-M, Braddock DT, Albright RA, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506). https://doi.org/10.1038/nature13270.
Madiraju AK, Qiu Y, Perry RJ, Rahimi Y, Zhang X-M, Zhang D, et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med. 2018;24:1–11. https://doi.org/10.1038/s41591-018-0125-4.
Funding
This work was financially supported by the NIH grants (R01DK081418, R01DK089883, and R01DK117655) to P.P, The Chares King Postdoctoral Fellowship to K.S, and the American Diabetes Association (ADA) Postdoctoral Fellowship to C.D.J.T.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Kfir Sharabi and Pere Puigserver have a pending patent to DFCI on selective inhibition of gluconeogenic activity.
Clint D. J. Tavares declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pathogenesis of Type 2 Diabetes and Insulin Resistance
Rights and permissions
About this article
Cite this article
Sharabi, K., Tavares, C.D.J. & Puigserver, P. Regulation of Hepatic Metabolism, Recent Advances, and Future Perspectives. Curr Diab Rep 19, 98 (2019). https://doi.org/10.1007/s11892-019-1224-4
Published:
DOI: https://doi.org/10.1007/s11892-019-1224-4