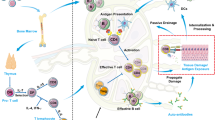
Abstract
Purpose of Review
This review seeks to characterize emerging concepts related to disease-modifying therapy in type 1 diabetes.
Recent Findings
We begin by describing the new understanding that islet autoimmunity, as identified by the presence of islet autoantibodies, inevitably leads to clinical type 1 diabetes. This understanding informs the new staging paradigm for type 1 diabetes, which suggests that type 1 diabetes may be recognized and diagnosed long before symptoms develop. Although it is known that nearly all individuals with established islet autoimmunity will eventually develop symptomatic type 1 diabetes (T1D), individual characteristics such as age and biomarker profile may predict rate of disease progression and response to treatment and may therefore be used to individualize therapy.
Summary
Key research supports the use of immunotherapy in TID, although a paradigm shift is necessary before immunotherapy may transition from clinical trials to clinical practice. Recent and ongoing research as it relates to these concepts is described throughout.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med. 1988;319(10):599–604.
Bougneres PF, Carel JC, Castano L, Boitard C, Gardin JP, Landais P, et al. Factors associated with early remission of type I diabetes in children treated with cyclosporine. N Engl J Med. 1988;318(11):663–70.
Stiller CR, Laupacis A, Dupre J, Jenner MR, Keown PA, Rodger W, et al. Cyclosporine for treatment of early type I diabetes: preliminary results. N Engl J Med. 1983;308(20):1226–7.
Type 1 diabetes: stage 1. [Image on the Internet]. C2017. Available from https://trialnet.org/t1d-facts/stages-of-t1d/stage-1.
•• Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964–74. This scientific statement redefined diagnostic criteria for T1D, suggesting that individuals found to have multiple autoantibodies may be characterized as being in “Stage 1” of the disease. Almost all individuals in stage 1 of T1D will eventually progress to symptomatic diabetes.
Ziegler AG, Rewers M, Eisenbarth G, Simell O. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. J Am Med Assoc. 2013;309(23):2473–9.
• Wherrett DK, Chiang JL, Delamater AM, DiMeglio LA, Gitelman SE, Gottlieb PA, et al. Defining pathways for development of disease-modifying therapies in children with type 1 diabetes: a consensus report. Diabetes Care. 2015;38(10):1975–85. This study shows that the risk of progression from autoantibody positivity to stage 3 (symptomatic) T1D varies based on age, with those at younger ages at greater risk of rapid progression of disease. This may justify the use of different therapies for the pediatric at-risk population versus the adult population.
Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, et al. The TrialNet natural history study of the development of type 1 diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10(2):97–104.
American Diabetes Association I. American Diabetes Association standards of medical Care in Diabetes 2017. Diabetes Care. 2017;40(Supplement 1):S1–S134.
Kostraba JN, Gay EC, Cai Y, Cruickshanks KJ, Rewers MJ, Klingensmith GJ, et al. Incidence of insulin-dependent diabetes mellitus in Colorado. Epidemiology. 1992;3(3):232–8.
Group EAS, Group EASS. Familial risk of type I diabetes in European children. Diabetologia. 1998;41(10):1151–6.
Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, Erlich HA. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet. 1996;59(5):1134.
Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360(16):1646–54.
Hagopian S, Erlich H, Lernmark A, JinXiong S. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011;12(8):133–43.
Greenbaum CJ, Beam CA, Boulware D, Gitelman SE, Gottlieb PA, Herold KC, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite type 1 diabetes TrialNet data. Diabetes. 2012;61(8):2066–73.
Parikka V, Näntö-Salonen K, Saarinen M, Simell T, Ilonen J, Hyöty H, et al. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia. 2012;55(7):1926–36.
Insel RA, Dunne JL, Ziegler AG. General population screening for type 1 diabetes: has its time come? Curr Opin Endocrinol Diabetes Obes. 2015;22(4):270–6.
TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8(5):286–98.
Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–9.
Orban T, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37(4):1069–75.
Pescovitz MD, Greenbaum CJ, Bundy B, Becker DJ, Gitelman SE, Goland R, et al. B-lymphocyte depletion with rituximab and beta-cell function: two-year results. Diabetes Care. 2014;37(2):453–9.
Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Rosenthal SM, Shuster JJ, et al. Anti-thymocyte globulin/G-CSF treatment preserves beta cell function in patients with established type 1 diabetes. J Clin Invest. 2015;125(1):448–55.
Gitelman SG, Gottlieb PA, Rigby MR, et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1(4):306–16.
Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–52.
Hagopian W, Ferry RJ, Sherry N, Carlin D, Bonvini E, Johnson S, et al. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized placebo-controlled Protégé trial. Diabetes. 2013;62(11):3901–8.
Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125(8):3285–96.
Sosenko JM, Skyler JS, Palmer JP, Krische JP, Liping Y, Mahon J, et al. The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care 2013;36(9):2615–20.
Vehik K, Cuthbertson D, Ruhlig H, Schatz DA, Peakman M, Krischer JP, et al. Long-term outcome of individuals treated with oral insulin: diabetes prevention trial-type 1 (DPT-1) oral insulin trial. Diabetes Care. 2011;34(7):1585–90.
Skyler J. Update on worldwide efforts to prevent type 1 diabetes. Ann N Y Acad Sci. 2008;1150:190.
(NIDDK) NIoDaDaKD. Oral insulin for prevention of diabetes in relatives at risk for type 1 diabetes mellitus. In: ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US) 2000- [cited 2017 Mar 31].Available from: https://clinicaltrials.gov/show/NCT00419562NLMIdentifier:NCT00419562.
Bottazzo G, Lorin-Christensen A, Doniach D. Islet cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–83.
Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, et al. Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132(2):166–73.
Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62(11):3766–74.
Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56(2):391–400.
Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352(25):2598–608.
Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53(4):614–23.
Rigby MR, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1(4):284–94.
Group DPTS. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685–91.
Sosenko JMPJ, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Matheson D, Skyler JS. Glucose and C-peptide changes in the perionset period of type 1 diabetes in the diabetes prevention trial-type 1. Diabetes Care. 2008;31(11):2188–92.
(NIDDK) NIoDaDaKD. Teplizumab for prevention of type 1 diabetes in relatives “at-risk”. In: ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US) 2000- [cited 2017 Mar 31].Available from: http://clinicaltrials.gov/show/ NCT01030861 NLM Identifier: NCT01030861.
(NIDDK) NIoDaDaKD. ATG-GCSF in new onset type 1 diabetes. In: ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US) 2000- [cited 2017 March 31].Available from: http://clinicaltrials.gov/show/ NCT02215200 NLM Identifier:NCT02215200.
Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803–13.
Health M. Trial of intranasal insulin in children and young adults at risk of type 1 diabetes (INITII). ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US) 2000- [cited 2017 March 31] Available from: http://clinicaltrials.gov/show/ NCT00336674 NLM Identifier:NCT00336674.
University L. Diabetes Prevention - Immune Tolerance (DIAPREV-IT). ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US) 2000- [cited 2017 April 13] Available from: http://clinicaltrials.gov/show/ NCT01122446 NLM Identifier: NCT01122446.
University L. Prevention Trial: Immune-tolerance With Alum-GAD (Diamyd) and vitamin D3 to Children With Multiple Islet Autoantibodies (DiAPREV-IT2). ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US) 2000- [cited 2017 April 13] Available from: http://clinicaltrials.gov/show/ NCT02387164 NLM Identifier: NCT02387164.
(NIDDK) NIoDaDaKD. CTLA4-Ig (abatacept) for prevention of abnormal glucose tolerance and diabetes in relatives at-risk for type 1. In: ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US) 2000- [cited 2017 March 31].Available from: http://clinicaltrials.gov/show/ NCT01773707 NLM Identifier:NCT01773707.
Bluestone J. T1DM Immunotherapy Using Polyclonal Tregs + IL-2 (TILT). In: ClinicalTrialsgov [Internet] Bethesda (MD): National Library of Medicine (US) 2000- [cited 2017 April 13] Available from: http://clinicaltrials.gov/show/ NCT02772679 NLM Identifier: NCT02772679.
Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLos One. 2010;5(7):e11501.
Miller RG, Secrest A, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburge Epidemiology of Diabetes Complications study cohort. Diabetes. 2012;61(11):2987–92.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Carla Greenbaum reports consultant fees from Lilly, grants and consultant fees from NovoNordisk, consultant fees from Bristol Myers Squibb, grants and consultant fees from Janssen, and consultant fees from Pfizer.
Sandra Lord and Dana VanBuecken declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Therapies and New Technologies in the Treatment of Type 1 Diabetes
Rights and permissions
About this article
Cite this article
Greenbaum, C., Lord, S. & VanBuecken, D. Emerging Concepts on Disease-Modifying Therapies in Type 1 Diabetes. Curr Diab Rep 17, 119 (2017). https://doi.org/10.1007/s11892-017-0932-x
Published:
DOI: https://doi.org/10.1007/s11892-017-0932-x