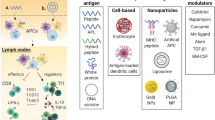
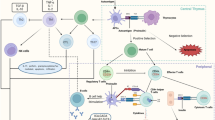
Abstract
Purpose of Review
The current standard therapy for type 1 diabetes (T1D) is insulin replacement. Autoimmune diseases are typically treated with broad immunosuppression, but this has multiple disadvantages. Induction of antigen-specific tolerance is preferable. The application of nanomedicine to the problem of T1D can take different forms, but one promising way is the development of tolerogenic nanoparticles, the aim of which is to mitigate the islet-destroying autoimmunity. We review the topic and highlight recent strategies to produce tolerogenic nanoparticles for the purpose of treating T1D.
Recent Findings
Several groups are making progress in applying tolerogenic nanoparticles to rodent models of T1D, while others are using nanotechnology to aid other potential T1D treatments such as islet transplant and islet encapsulation.
Summary
The strategies behind how nanoparticles achieve tolerance are varied. It is likely the future will see even greater diversity in tolerance induction strategies as well as a greater focus on how to translate this technology from preclinical use in mice to treatment of T1D in humans.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
In’t VP. Insulitis in human type 1 diabetes: a comparison between patients and animal models. Semin Immunopathol. 2014;36(5):569–79. doi:10.1007/s00281-014-0438-4.
Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14(1):45–57. doi:10.1038/nrd4477.
Kyi M, Wentworth JM, Nankervis AJ, Fourlanos S, Colman PG. Recent advances in type 1 diabetes. Med J Aust. 2015;203(7):290–3.
McCarthy DP, Hunter ZN, Chackerian B, Shea LD, Miller SD. Targeted immunomodulation using antigen-conjugated nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(3):298–315. doi:10.1002/wnan.1263.
• Getts DR, Shea LD, Miller SD, King NJ. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015;36(7):419–27. doi:10.1016/j.it.2015.05.007. A comprehensive review on the use of nanoparticles for immune modulation and tolerance.
Serra P, Santamaria P. Nanoparticle-based autoimmune disease therapy. Clin Immunol. 2015;160(1):3–13. doi:10.1016/j.clim.2015.02.003.
Hanninen A, Jalkanen S, Salmi M, Toikkanen S, Nikolakaros G, Simell O. Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J Clin Invest. 1992;90(5):1901–10. doi:10.1172/JCI116067.
Lernmark A, Kloppel G, Stenger D, Vathanaprida C, Falt K, Landin-Olsson M, et al. Heterogeneity of islet pathology in two infants with recent onset diabetes mellitus. Virchows Arch. 1995;425(6):631–40.
Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155(2):173–81. doi:10.1111/j.1365-2249.2008.03860.x.
Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209(1):51–60. doi:10.1084/jem.20111187.
Eisenbarth GS. Banting lecture 2009: an unfinished journey: molecular pathogenesis to prevention of type 1A diabetes. Diabetes. 2010;59(4):759–74. doi:10.2337/db09-1855.
Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(Suppl 2):S52–61.
Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104(43):17040–5. doi:10.1073/pnas.0705894104.
Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, et al. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes. 2008;57(10):2693–7. doi:10.2337/db08-0522.
Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222(4630):1337–9.
Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347(6289):151–6. doi:10.1038/347151a0.
Lampasona V, Bearzatto M, Genovese S, Bosi E, Ferrari M, Bonifacio E. Autoantibodies in insulin-dependent diabetes recognize distinct cytoplasmic domains of the protein tyrosine phosphatase-like IA-2 autoantigen. J Immunol. 1996;157(6):2707–11.
Bingley PJ, Christie MR, Bonifacio E, Bonfanti R, Shattock M, Fonte MT, et al. Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes. 1994;43(11):1304–10.
Skyler JS, Ricordi C. Stopping type 1 diabetes: attempts to prevent or cure type 1 diabetes in man. Diabetes. 2011;60(1):1–8. doi:10.2337/db10-1114.
Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi:10.1016/S0140-6736(13)60591-7.
Vives M, Somoza N, Soldevila G, Gomis R, Lucas A, Sanmarti A, et al. Reevaluation of autoantibodies to islet cell membrane in IDDM. Failure to detect islet cell surface antibodies using human islet cells as substrate. Diabetes. 1992;41(12):1624–31.
Koczwara K, Bonifacio E, Ziegler AG. Transmission of maternal islet antibodies and risk of autoimmune diabetes in offspring of mothers with type 1 diabetes. Diabetes. 2004;53(1):1–4.
Pathiraja V, Kuehlich JP, Campbell PD, Krishnamurthy B, Loudovaris T, Coates PT, et al. Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes. 2015;64(1):172–82. doi:10.2337/db14-0858.
Skowera A, Ellis RJ, Varela-Calvino R, Arif S, Huang GC, Van-Krinks C, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118(10):3390–402. doi:10.1172/JCI35449.
Panina-Bordignon P, Lang R, van Endert PM, Benazzi E, Felix AM, Pastore RM, et al. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J Exp Med. 1995;181(5):1923–7.
Unger WW, Pearson T, Abreu JR, Laban S, van der Slik AR, der Kracht SM, et al. Islet-specific CTL cloned from a type 1 diabetes patient cause beta-cell destruction after engraftment into HLA-A2 transgenic NOD/scid/IL2RG null mice. PLoS One. 2012;7(11):e49213. doi:10.1371/journal.pone.0049213.
Mannering SI, Pathiraja V, Kay TW. The case for an autoimmune aetiology of type 1 diabetes. Clin Exp Immunol. 2016;183(1):8–15. doi:10.1111/cei.12699.
•• Babon JA, ME DN, Blodgett DM, Crevecoeur I, Buttrick TS, Maehr R, et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med. 2016;22(12):1482–7. doi:10.1038/nm.4203. The first comprehensive description of specificity of islet-infiltrating T cells from human donors with T1D.
Jayasimhan A, Mansour KP, Slattery RM. Advances in our understanding of the pathophysiology of type 1 diabetes: lessons from the NOD mouse. Clin Sci (Lond). 2014;126(1):1–18. doi:10.1042/CS20120627.
Pearson JA, Wong FS, Wen L. The importance of the non obese diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun. 2016;66:76–88. doi:10.1016/j.jaut.2015.08.019.
•• Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435(7039):220–3. Genetic evidence indicating that an autoimmune response to an insulin epitope drives development of T1D in NOD mice.
•• Prasad S, Kohm AP, JS MM, Luo X, Miller SD. Pathogenesis of NOD diabetes is initiated by reactivity to the insulin B chain 9-23 epitope and involves functional epitope spreading. J Autoimmun. 2012;39(4):347–53. doi:10.1016/j.jaut.2012.04.005. Tolerogenic evidence that an autoimmune response to a dominant insulin epitope drives development of T1D in NOD mice and evidence for epitope spreading as disease progresses.
Babad J, Geliebter A, DiLorenzo TP. T-cell autoantigens in the non-obese diabetic mouse model of autoimmune diabetes. Immunology. 2010;131(4):459–65. doi:10.1111/j.1365-2567.2010.03362.x.
• Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity, 2010. 32(4):488–99. doi:10.1016/j.immuni.2010.04.002. A comprehensive review of immune-based clinical trials in T1D patients.
Berry G, Waldner H. Accelerated type 1 diabetes induction in mice by adoptive transfer of diabetogenic CD4+ T cells. J Vis Exp. 2013;75:e50389. doi:10.3791/50389.
Presa M, Chen YG, Grier AE, Leiter EH, Brehm MA, Greiner DL, et al. The presence and preferential activation of regulatory T cells diminish adoptive transfer of autoimmune diabetes by polyclonal nonobese diabetic (NOD) T cell effectors into NSG versus NOD-scid mice. J Immunol. 2015;195(7):3011–9. doi:10.4049/jimmunol.1402446.
Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39(1):216–24. doi:10.1002/eji.200838475.
Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31(4):654–64. doi:10.1016/j.immuni.2009.08.023.
Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37(10):1444–8.
Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74(6):1089–100.
Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11(3):225–31. doi:10.1038/ni.1844.
Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sorensen SJ, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55(8):2285–94. doi:10.1007/s00125-012-2564-7.
Xu D, Prasad S, Miller SD. Inducing immune tolerance: a focus on type 1 diabetes mellitus. Diabetes Manag (Lond). 2013;3(5):415–26. doi:10.2217/dmt.13.36.
Michels AW, Eisenbarth GS. Immune intervention in type 1 diabetes. Semin Immunol. 2011;23(3):214–9. doi:10.1016/j.smim.2011.07.003.
Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–300.
Luo X, Miller SD, Shea LD. Immune tolerance for autoimmune disease and cell transplantation. Annu Rev Biomed Eng. 2016;18:181–205. doi:10.1146/annurev-bioeng-110315-020137.
Plesner A, Worsaae A, Dyrberg T, Gotfredsen C, Michelsen BK, Petersen JS. Immunization of diabetes-prone or non-diabetes-prone mice with GAD65 does not induce diabetes or islet cell pathology. J Autoimmun. 1998;11(4):335–41. doi:10.1006/jaut.1998.0206.
Uibo R, Lernmark A. GAD65 autoimmunity-clinical studies. Adv Immunol. 2008;100:39–78. doi:10.1016/S0065-2776(08)00803-1.
Agardh CD, Cilio CM, Lethagen A, Lynch K, Leslie RD, Palmer M, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complicat. 2005;19(4):238–46. doi:10.1016/j.jdiacomp.2004.12.003.
Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359(18):1909–20. doi:10.1056/NEJMoa0804328.
Ludvigsson J, Hjorth M, Cheramy M, Axelsson S, Pihl M, Forsander G, et al. Extended evaluation of the safety and efficacy of GAD treatment of children and adolescents with recent-onset type 1 diabetes: a randomised controlled trial. Diabetologia. 2011;54(3):634–40. doi:10.1007/s00125-010-1988-1.
Cheramy M, Skoglund C, Johansson I, Ludvigsson J, Hampe CS, Casas R. GAD-alum treatment in patients with type 1 diabetes and the subsequent effect on GADA IgG subclass distribution, GAD65 enzyme activity and humoral response. Clin Immunol. 2010;137(1):31–40. doi:10.1016/j.clim.2010.06.001.
Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378(9788):319–27. doi:10.1016/S0140-6736(11)60895-7.
Harrison LC, Honeyman MC, Steele CE, Stone NL, Sarugeri E, Bonifacio E, et al. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004;27(10):2348–55.
Fourlanos S, Perry C, Gellert SA, Martinuzzi E, Mallone R, Butler J, et al. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes. 2011;60(4):1237–45. doi:10.2337/db10-1360.
Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the diabetes prevention trial—type 1. Diabetes Care. 2005;28(5):1068–76.
Skyler JS, Greenbaum CJ, Lachin JM, Leschek E, Rafkin-Mervis L, Savage P, et al. Type 1 diabetes TrialNet—an international collaborative clinical trials network. Ann N Y Acad Sci. 2008;1150:14–24. doi:10.1196/annals.1447.054.
Pozzilli P, Pitocco D, Visalli N, Cavallo MG, Buzzetti R, Crino A, et al. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB group. Diabetologia. 2000;43(8):1000–4.
Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203(12):2737–47. doi:10.1084/jem.20061577.
Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med. 2011;208(7):1501–10. doi:10.1084/jem.20110574.
Judkowski V, Rodriguez E, Pinilla C, Masteller E, Bluestone JA, Sarvetnick N, et al. Peptide specific amelioration of T cell mediated pathogenesis in murine type 1 diabetes. Clin Immunol. 2004;113(1):29–37. doi:10.1016/j.clim.2004.03.007.
Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204(1):191–201. doi:10.1084/jem.20061631.
Weber SE, Harbertson J, Godebu E, Mros GA, Padrick RC, Carson BD, et al. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J Immunol. 2006;176(8):4730–9.
Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54(2):306–10.
• Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7(315):315ra189. doi:10.1126/scitranslmed.aad4134. Description of the use of Tregs for therapy of T1D.
Xia CQ, Peng R, Qiu Y, Annamalai M, Gordon D, Clare-Salzler MJ. Transfusion of apoptotic beta-cells induces immune tolerance to beta-cell antigens and prevents type 1 diabetes in NOD mice. Diabetes. 2007;56(8):2116–23. doi:10.2337/db06-0825.
Marin-Gallen S, Clemente-Casares X, Planas R, Pujol-Autonell I, Carrascal J, Carrillo J, et al. Dendritic cells pulsed with antigen-specific apoptotic bodies prevent experimental type 1 diabetes. Clin Exp Immunol. 2010;160(2):207–14. doi:10.1111/j.1365-2249.2009.04082.x.
Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–13. doi:10.1002/eji.200737984.
Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173(5):3148–54.
Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, Hubbell JA, et al. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLoS One. 2013;8(4):e61646. doi:10.1371/journal.pone.0061646.
Garciafigueroa Y, Trucco M, Giannoukakis N. A brief glimpse over the horizon for type 1 diabetes nanotherapeutics. Clin Immunol. 2015;160(1):36–45. doi:10.1016/j.clim.2015.03.016.
Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9(1):16–30. doi:10.1021/nn5062029.
Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–70. doi:10.1038/nbt1340.
Kwon YJ, Standley SM, Goh SL, Frechet JM. Enhanced antigen presentation and immunostimulation of dendritic cells using acid-degradable cationic nanoparticles. J Control Release. 2005;105(3):199–212. doi:10.1016/j.jconrel.2005.02.027.
Murthy N, Xu M, Schuck S, Kunisawa J, Shastri N, Frechet JM. A macromolecular delivery vehicle for protein-based vaccines: acid-degradable protein-loaded microgels. Proc Natl Acad Sci U S A. 2003;100(9):4995–5000. doi:10.1073/pnas.0930644100.
Fromen CA, Robbins GR, Shen TW, Kai MP, Ting JP, DeSimone JM. Controlled analysis of nanoparticle charge on mucosal and systemic antibody responses following pulmonary immunization. Proc Natl Acad Sci U S A. 2015;112(2):488–93. doi:10.1073/pnas.1422923112.
Bal SM, Hortensius S, Ding Z, Jiskoot W, Bouwstra JA. Co-encapsulation of antigen and toll-like receptor ligand in cationic liposomes affects the quality of the immune response in mice after intradermal vaccination. Vaccine. 2011;29(5):1045–52. doi:10.1016/j.vaccine.2010.11.061.
Koppolu B, Zaharoff DA. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials. 2013;34(9):2359–69. doi:10.1016/j.biomaterials.2012.11.066.
Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target. 2004;12(9–10):635–41. doi:10.1080/10611860400015936.
Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, et al. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65(1–2):133–48.
Pensado A, Fernandez-Pineiro I, Seijo B, Sanchez A. Anionic nanoparticles based on span 80 as low-cost, simple and efficient non-viral gene-transfection systems. Int J Pharm. 2014;476(1–2):23–30. doi:10.1016/j.ijpharm.2014.09.032.
Ruenraroengsak P, Tetley TD. Differential bioreactivity of neutral, cationic and anionic polystyrene nanoparticles with cells from the human alveolar compartment: robust response of alveolar type 1 epithelial cells. Part Fibre Toxicol. 2015;12:19. doi:10.1186/s12989-015-0091-7.
Liu Y, Li W, Lao F, Liu Y, Wang L, Bai R, et al. Intracellular dynamics of cationic and anionic polystyrene nanoparticles without direct interaction with mitotic spindle and chromosomes. Biomaterials. 2011;32(32):8291–303. doi:10.1016/j.biomaterials.2011.07.037.
Tagalakis AD, Lee DH, Bienemann AS, Zhou H, Munye MM, Saraiva L, et al. Multifunctional, self-assembling anionic peptide-lipid nanocomplexes for targeted siRNA delivery. Biomaterials. 2014;35(29):8406–15. doi:10.1016/j.biomaterials.2014.06.003.
Sun Y, Guo F, Zou Z, Li C, Hong X, Zhao Y, et al. Cationic nanoparticles directly bind angiotensin-converting enzyme 2 and induce acute lung injury in mice. Part Fibre Toxicol. 2015;12:4. doi:10.1186/s12989-015-0080-x.
Yang SH, Heo D, Park J, Na S, Suh JS, Haam S, et al. Role of surface charge in cytotoxicity of charged manganese ferrite nanoparticles towards macrophages. Nanotechnology. 2012;23(50):505702. doi:10.1088/0957-4484/23/50/505702.
Chao Y, Makale M, Karmali PP, Sharikov Y, Tsigelny I, Merkulov S, et al. Recognition of dextran-superparamagnetic iron oxide nanoparticle conjugates (Feridex) via macrophage scavenger receptor charged domains. Bioconjug Chem. 2012;23(5):1003–9. doi:10.1021/bc200685a.
Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103(13):4930–4. doi:10.1073/pnas.0600997103.
Verma A, Uzun O, Hu Y, Hu Y, Han HS, Watson N, et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat Mater. 2008;7(7):588–95. doi:10.1038/nmat2202.
Stone JW, Thornburg NJ, Blum DL, Kuhn SJ, Wright DW, Crowe JE Jr. Gold nanorod vaccine for respiratory syncytial virus. Nanotechnology. 2013;24(29):295102. doi:10.1088/0957-4484/24/29/295102.
Karch CP, Li J, Kulangara C, Paulillo SM, Raman SK, Emadi S, et al. Vaccination with self-adjuvanted protein nanoparticles provides protection against lethal influenza challenge. Nanomedicine. 2017;13(1):241–51. doi:10.1016/j.nano.2016.08.030.
Doll TA, Neef T, Duong N, Lanar DE, Ringler P, Muller SA, et al. Optimizing the design of protein nanoparticles as carriers for vaccine applications. Nanomedicine. 2015;11(7):1705–13. doi:10.1016/j.nano.2015.05.003.
Babapoor S, Neef T, Mittelholzer C, Girshick T, Garmendia A, Shang H, et al. A novel vaccine using nanoparticle platform to present immunogenic M2e against avian influenza infection. Influenza Res Treat. 2011;2011:126794. doi:10.1155/2011/126794.
Wahome N, Pfeiffer T, Ambiel I, Yang Y, Keppler OT, Bosch V, et al. Conformation-specific display of 4E10 and 2F5 epitopes on self-assembling protein nanoparticles as a potential HIV vaccine. Chem Biol Drug Des. 2012;80(3):349–57. doi:10.1111/j.1747-0285.2012.01423.x.
Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7(12):779–86. doi:10.1038/nnano.2012.207.
Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8(10):772–81. doi:10.1038/nnano.2013.181.
Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A. 2008;105(38):14265–70. doi:10.1073/pnas.0805135105.
• Smarr CB, Yap WT, Neef TP, Pearson RM, Hunter ZN, Ifergan I, et al. Biodegradable antigen-associated PLG nanoparticles tolerize Th2-mediated allergic airway inflammation pre- and postsensitization. Proc Natl Acad Sci USA. 2016;113(18):5059–64. doi:10.1073/pnas.1505782113. Demonstration that infusion of antigen-encapsulating PLG nanoparticles induce tolerance in Th2 cells useful in prevention and treatment in an animal model of allergic airway disease.
Ilinskaya AN, Dobrovolskaia MA. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br J Pharmacol. 2014;171(17):3988–4000. doi:10.1111/bph.12722.
Phillips B, Nylander K, Harnaha J, Machen J, Lakomy R, Styche A, et al. A microsphere-based vaccine prevents and reverses new-onset autoimmune diabetes. Diabetes. 2008;57(6):1544–55. doi:10.2337/db07-0507.
Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29(11):1005–10. doi:10.1038/nbt.1989.
Wang P, Yigit MV, Ran C, Ross A, Wei L, Dai G, et al. A theranostic small interfering RNA nanoprobe protects pancreatic islet grafts from adoptively transferred immune rejection. Diabetes. 2012;61(12):3247–54. doi:10.2337/db12-0441.
Shah M, Edman MC, Janga SR, Shi P, Dhandhukia J, Liu S, et al. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjogren’s syndrome. J Control Release. 2013;171(3):269–79. doi:10.1016/j.jconrel.2013.07.016.
Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32(4):568–80. doi:10.1016/j.immuni.2010.03.015.
•• Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530(7591):434–40. doi:10.1038/nature16962. Demonstration that iron oxide nanoparticles expressing peptide-MHC molecules can induce tolerance for treatment of autoimmune disease.
Singha S, Shao K, Yang Y, Clemente-Casares X, Sole P, Clemente A, et al. Peptide-MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat Nanotechnol. 2017; doi:10.1038/nnano.2017.56.
Schutz C, Fleck M, Mackensen A, Zoso A, Halbritter D, Schneck JP, et al. Killer artificial antigen-presenting cells: a novel strategy to delete specific T cells. Blood. 2008;111(7):3546–52. doi:10.1182/blood-2007-09-113522.
Schutz C, Fleck M, Schneck JP, Oelke M. Killer artificial antigen presenting cells (KaAPC) for efficient in vitro depletion of human antigen-specific T cells. J Vis Exp. 2014;90:e51859. doi:10.3791/51859.
Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A. 2015;112(2):E156–65. doi:10.1073/pnas.1408686111.
Zhang AH, Rossi RJ, Yoon J, Wang H, Scott DW. Tolerogenic nanoparticles to induce immunologic tolerance: prevention and reversal of FVIII inhibitor formation. Cell Immunol. 2016;301:74–81. doi:10.1016/j.cellimm.2015.11.004.
Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2012;109(28):11270–5. doi:10.1073/pnas.1120611109.
•• Yeste A, Takenaka MC, Mascanfroni ID, Nadeau M, Kenison JE, Patel B, et al. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci Signal. 2016;9(433):ra61. doi:10.1126/scisignal.aad0612. Demonstration that gold nanoparticles expressing antigen and an AhR agonist can prevent development of T1D in NOD mice.
Macauley MS, Pfrengle F, Rademacher C, Nycholat CM, Gale AJ, von Drygalski A, et al. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J Clin Invest. 2013;123(7):3074–83. doi:10.1172/JCI69187.
Pfrengle F, Macauley MS, Kawasaki N, Paulson JC. Copresentation of antigen and ligands of Siglec-G induces B cell tolerance independent of CD22. J Immunol. 2013;191(4):1724–31. doi:10.4049/jimmunol.1300921.
Copp JA, Fang RH, Luk BT, Hu CM, Gao W, Zhang K, et al. Clearance of pathological antibodies using biomimetic nanoparticles. Proc Natl Acad Sci U S A. 2014;111(37):13481–6. doi:10.1073/pnas.1412420111.
•• Getts DR, Martin AJ, DP MC, Terry RL, Hunter ZN, Yap WT, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30(12):1217–24. doi:10.1038/nbt.2434. Initial description that antigen coupled to polystyrene and PLG nanoparticles can induce tolerance active in prevention of EAE.
•• Hunter Z, DP MC, Yap WT, Harp CT, Getts DR, Shea LD, et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8(3):2148–60. doi:10.1021/nn405033r. Description of the ability of antigen-coupled PLG nanoparticles to treat ongoing EAE.
•• McCarthy DP, Yap JW, Harp CT, Song WK, Chen J, Pearson RM, et al. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomedicine. 2017;13(1):191–200. doi:10.1016/j.nano.2016.09.007. Description of the ability of antigen-encapsulating PLG nanoparticles to prevent and treat EAE.
• Hlavaty KA, DP MC, Saito E, Yap WT, Miller SD, Shea LD. Tolerance induction using nanoparticles bearing HY peptides in bone marrow transplantation. Biomaterials. 2016;76:1–10. doi:10.1016/j.biomaterials.2015.10.041. Description of the ability of either antigen-coupled or antigen encapsulating nanoparticles to prevent bone marrow transplant rejection.
•• Miller SD, Prasad S, Neef T. Antigen-encapsulating PLG nanoparticles induce long-lived regulatory T cell control of activated diabetogenic CD4 and CD8 T cells (Abstract). Immunology of Diabetes Society 15th International Congress. 2017. Demonstration of nanoparticle tolerance for the treatment of adoptive transfer models of T1D.
• Bryant J, Hlavaty KA, Zhang X, Yap WT, Zhang L, Shea LD, et al. Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation. Biomaterials. 2014;35(31):8887–94. doi:10.1016/j.biomaterials.2014.06.044. Demonstration that antigen-coupled nanoparticles can induce tolerance to protect transplanted allogeneic islets.
Hlavaty KA, Luo X, Shea LD, Miller SD. Cellular and molecular targeting for nanotherapeutics in transplantation tolerance. Clin Immunol. 2015;160(1):14–23. doi:10.1016/j.clim.2015.03.013.
Chopra S, Bertrand N, Lim JM, Wang A, Farokhzad OC, Karnik R. Design of insulin-loaded nanoparticles enabled by multistep control of nanoprecipitation and zinc chelation. ACS Appl Mater Interfaces. 2017;9(13):11440–50. doi:10.1021/acsami.6b16854.
• Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105(38):14527–32. doi:10.1073/pnas.0805204105. Description of the ability of tolerance induced by the i.v. infusion of apoptotic donor leukocytes to long-term survival of transplanted donor islets.
Desai T, Shea LD. Advances in islet encapsulation technologies. Nat Rev Drug Discov. 2016; doi:10.1038/nrd.2016.232.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tobias Neef declares that he has no conflict of interest.
Stephen D. Miller reports grants and personal fees from Cour Pharmaceutical Development Co. In addition, Dr. Miller has a patent US 14/410,011 issued.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
This article is part of the Topical Collection on Pathogenesis of Type 1 Diabetes
Rights and permissions
About this article
Cite this article
Neef, T., Miller, S.D. Tolerogenic Nanoparticles to Treat Islet Autoimmunity. Curr Diab Rep 17, 84 (2017). https://doi.org/10.1007/s11892-017-0914-z
Published:
DOI: https://doi.org/10.1007/s11892-017-0914-z