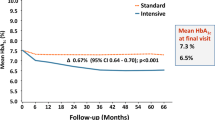
Abstract
Purpose of Review
There are currently over 40 different drugs in 12 distinct classes approved in the USA to treat patients with type 2 diabetes mellitus. This review summarizes our current knowledge about potential side effects of antidiabetic therapy and attempts to apply it to a clinical practice setting.
Recent Findings
Given the heterogeneity of both the patients and the disease, it is mathematically impossible to test every available drug combination in long-term outcome, prospective, randomized blinded fashion before a clinician decides which agent(s) to prescribe to a specific patient in a given situation. To complicate the clinician’s dilemma, there is lack of available tests to predict an individual’s response or propensity to side effects. Further, the data available are derived from small, short-term registration trials and typically focus on relative rather than absolute risks of any given drug and do not address the potential adverse outcomes if a patient’s diabetes remains untreated.
Summary
Clinicians have to personalize their choice of antidiabetic therapy based both on the specific characteristics of the patient in front of them (stage of diabetes and its complications, overall health status, socioeconomic situation, other medications present, desire to improve control of diabetes, etc.) and the current knowledge about the relative and absolute balance of benefits and risks of any individual medication in that specific patient. It has to be recognized that this requires constant re-evaluation as database of our experience with antidiabetic therapy expands.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015. A patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diab Care. 2015;(38):140–9.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract. 2016;(22):84–113.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71.
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109026.htm (Avandia restrictions)
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm376516.htm (Avandia restriction lifted)
Dormandy JA, Charbonnel B, Eckland DJA, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (prospective pioglitazone clinical trial in macrovascular events): a randomised controlled trial. Lancet. 2005;366:1279–89.
Neumann A, Weill A, Ricordeau P, Fagot JP, All F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55:1953–62.
Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry Jr CP, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone. Interim report of a longitudinal cohort study. Diab Care. 2011;34:916–22.
• Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA. 2015;314:265–77. This 10-year prospective study did not show increase in bladder cancer among pioglitazone users
Hemkens LG, Grouven U, Bender R, Günster C, Gutschmidt S, Selke GW, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–44.
Smith U, Gale EAM. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52:1699–708.
Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28.
Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of endocrine and exocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–604.
• Andersen DK, Andern-Sandberg A, Duell EJ, Goggins M, Korc M, Petersen GM, et al. Pancreatitis- diabetes- pancreatic cancer: summary of an NIDDK-NCI workshop. Pancreas. 2013;42:1227–37. No causal relationship between incretin enhancing drugs and pancreatic cancer
Egan AG, Blind E, Dunder K, de Graeff PA, Hummer TB, Bourcier T, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med. 2014;370:794–7.
• Marso SP, Daniels GH, Brown-Fradsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. Cardiovascular benefit of a GLP-1 receptor agonist
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jödar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med Set. 2016;16 doi:10.1056/NEJMoa1607141. Cardiovascular benefit of another GLP-1 receptor agonist
http://www.fda.gov/Drugs/DrugSafety/ucm505860.htm (warning on acute kidney injury with canagliflozin and dapagliflozin)
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. Possible renoprotection by an SGLT-2 inhibitor
Heerspink HJL, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2016;28 doi:10.1681/ASN.2016030278.
http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects. (FDA AERS)
http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm (FDA warning on SGLT-2i & DKA)
Data on file. Janssen Pharmaceuticals, Inc. Based on IMS Health, NPA Weekly, Total Prescriptions, April 2013–April 8th 2016.
Executive Summary – Mar. 15, 2011; Oral diabetes medications for adults with type 2 diabetes: an UpdateKey question 3: adverse events and side effects (AHRQ: Comparative Effectiveness Review Number 27); www.effectivehealthcare. ahrq.gov/reports/final.cfm .
Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147:386–99.
Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes. A systematic review and meta-analysis. Ann Int Med. 2016;164:740–51.
Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs on patients with type 2 diabetes. A meta-analysis JAMA. 2016;316:313–24.
•• Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. First reported study with an antidiabetic agent showing cardiovascular benefit
Blonde L, Dailey GE, Jabbour SA, Reasner CA, Mills DJ. Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: results of a retrospective cohort study. Curr Med Res Opin. 2004;20:565–72.
http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm (revised metformin warning)
Ekström N, Schiöler L, Svensson A-M, Eeg-Olofsson K, Jonasson JM, Zethelius B. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open. 2012;2:e001076.
Roomie CL, Hung AM, Greedy RA, Grijalva CG, Liu X, Murff HJ, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus. A cohort study. Ann Intern Med. 2012;157:601–10.
Meinert CL, Natterud GL, Prout TE, Klimt CR. A story of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II Mortality results Diabetes. 1970;19(Suppl):789–830.
Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning - is it time to retire glyburide? J Clin Endocrinol Metab. 2003;88:528–30.
Riddle MC. More reasons to day goodbye to glyburide. J Clin Endocrinol Metab. 2010;95:4867–70.
Mühlhauser I, Sawicki PT, Berger M. Possible risk of sulphonylureas in the treatment of non-insulin-dependent diabetes mellitus and coronary artery disease. Diabetologia. 1997;40:1492–3.
Zeller M, Danchin N, Simon D, Vahanian A, Lorgis L, Cotton Y. Impact of type of readmission sulfonylureas on mortality and cardiovascular outcomes diabetic patients with acute myocardial infarction. J Clin Endocrinol Metab. 2010;95:4993–5002.
Abdelmoneim AS, Eurich DT, Light PE, Senior PA, Seubert JM, Makowsky MJ, et al. Cardiovascular safety of sulphonylureas: over 40 years of continuous controversy without an answer. Diabetes Obes Metab. 2015;17:523–32.
Baxter CA, Das R, Langerman H, Mills EJ, Druyts E, Siliman G, et al. Increased risk of cardiovascular-related events associated with sulfonylureas compared to other antihyperglycaemic drugs: a Bayesian meta-analysis of survival data. Abstract 128; 51st annual meeting EASD 2015.
Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15:938–53.
Li Y, Hu Y, Ley S, Rajpathak S, Hu FB. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care. 2014;37:3106–13.
Forst T, Hanefeld M, Jacob S, MOeser G, Schwenk G, Pfützner A, et al. Association of sulphonylurea treatment with all-cause and cardiovascular mortality: a systematic review and meta-analysis of observation studies. Diab Vasc Dis Res. 2013;10:302–14.
Rados DV, Pinto LC, Remonti LR, Leitão CB, Gross JL. The association between sulfonylurea use and all-cause and cardiovascular mortality: a meta-analysis with trial sequential analysis of randomized clinical trials. PLoS Med. doi:10.1371/journal.pmed.1001992. April 12, 2016.
Johnson JA. The safety of sulfonylurea therapy in type 2 diabetes: have we reached the practical limits of our evidence base? Diabetologia. 2015;58:1–3.
Moses RG, Gomis R, Frandsen KB, Schlienger J-L, Dedov I. Flexible meal-related dosing with repaglinide facilitates glycemic control in therapy-naive type 2 diabetes. Diab Care. 2001;24:11–5.
Jovanovic L, Dailey III G, Huang WC, Strange P, Goldstein BJ. Repaglinide in type 2 diabetes: a 24-week, fixed-dose efficacy and safety study. J Clin Pharmacol. 2000;40:49–57.
Rosenstock J, Hassman DR, Madder RD, Shari A, Brazinsky SA, Farrell J, et al. Repaglinide versus nateglinide monotherapy. Diab Care. 2004;27:1265–70.
Lipska KJ, Yao X, Herrin J, McCoy RG, Ross JS, Steinman MA, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diab Care. 2016; Sep;dc160985 doi:10.2337/dc16-0985.
DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators, Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–105.
DeFronzo RA, Tripathy D, Schwenke DC, Banerji MA, Bray GA, Buchanan TA, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–15.
Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43.
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–31.
Grunberger G. Will PPAR-γ agonist therapy still have a role in diabetes management in 2013? Diabetes Manage. 2013;2:41–51.
Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ. 2012;184:E675–83.
Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med. 2012;172:1005–11.
Ambrosius WT, Danis RP, Goff Jr DC, Greven CM, Gerstein HC, Cohen RM, et al. Lack of association between thiazolidinediones and macular edema in type 2 diabetes: the ACCORD eye study. Arch Ophthalmol. 2010;128:312–8.
Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ. 2011;342:d1309.
Holman RR, Turner RC, Cull CA. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. prospective diabetes study 44). Diabetes Care. 1999;22:960–4.
Coniff RF, Shapiro JA, Seaton TB. Long-term efficacy and safety of acarbose in the treatment of obese subjects with non-insulin-dependent diabetes mellitus. Arch Intern Med. 1994;154:2442–8.
Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13:7–18.
Grunberger G. Clinical utility of dipeptidyl peptidase-4 inhibitors: a descriptive summary of current clinical efficacy trials. Eur J Clin Pharmacol. 2014;70:1277–89.
http://www.fda.gov/Drugs/DrugSafety PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm183764.htm. (sitagliptin - pancreatitis warning)
Girgis C, Champion B. Vildagliptin-induced acute pancreatitis. Endocr Pract. 2011;17:e48–50.
Kunjathaya P, Ramaswami PK, Krishnamurthy AN, Bhat N. Acute necrotizing pancreatitis associated with vildagliptin. J Pancreas. 2013;14:81–4.
Meier JJ, Nauck MA. Risk of pancreatitis in patients treated with incretin-based therapies. Diabetologia. 2014;57:1320–4.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
http://www.fda.gov/Drugs/DrugSafety/ucm486096.htm (FDA warning about heart failure and DPP-4i’s)
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35.
Fu AZ, Johnston SS, Ghannam A, Tsai K, Cappell K, Fowler R, et al. Association between hospitalization for heart failure and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes: an observational study. Diab Care. 2016;39:726–34.
Yu OHY, Filion KB, Azoulay L, Patenaude V, Majdan A, Suissa S. Incretin-based drugs and the risk of congestive heart failure. Diab Care. 2015;38:277–84.
Toh S, Hampp C, Reichman ME, Graham DJ, Balakrishnan S, Pucino F, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs. Ann Int Med. 2016;164:705–14.
Agersø H, Jensen LB, Elbrønd B, Rølan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202.
Grunberger G. Novel therapies for the management of type 2 diabetes mellitus: part 2. Addressing the incretin defect in the clinical setting in 2013. J of Diab. 2013;5:241–53.
Prada-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs in Context. 2015;4:212283.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57.
Azoulay L, Filion KB, Platt RW, Dahl M, Colin R, Dormuth CR, et al. Association between incretin-based drugs and the risk of acute pancreatitis. JAMA Intern Med. 2016;176:1464–73. No increased risk of acute pancreatitis by incretin enhancing drugs
Butler PC. Glucagon-like peptide 1 drugs as second-line therapy for type 2 diabetes. JAMA Intern Med. 2016; doi:10.1001/jamainternmed.2016.1523.
Cefalu WT, Buse JB, Del Prato S, Home PD, LeRoith D, Nauck MA, et al. Beyond metformin: safety consideration in the decision making process for selecting a second medication for type 2 diabetes management. Reflections from a diabetes care editors’ expert forum. Diab Care. 2014;37:2647–59.
Fonseca VA, Rosenstock J, Wang AC, Kenneth E. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diab Care. 2008;31:1479–84.
Goldfine AB, Fonseca VA, Jones MR, Wang AC, Ford DM, Truitt KE. Long-term safety and tolerability of colesevelam HCl in subjects with type 2 diabetes. Horm Metab Res. 2010;42:23–30.
Handelsman Y. Role of bile acid sequestrants in the treatment of type 2 diabetes. Diabetes Care. 2011;34(Suppl 2):S244–50.
Cincotta AH, Schiller BC, Meier AH. Bromocriptine inhibits the seasonally occurring obesity, hyperinsulinemia, insulin resistance, and impaired glucose tolerance in the Syrian hamster, Mesocricetus auratus. Metabolism. 1991;40:639–44.
Cincotta AH, Meier AH, Cincotta Jr M. Bromocriptine improves glycemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investing Drugs. 1999;8:1683–707.
Florez H, Scranton R, Farwell WR, DeFronzo RA, Ezrokhi M, Gaziano JM, et al. Randomized clinical trial assessing the efficacy and safety of bromocriptine-QR when added to ongoing thiazolidinedione therapy in patients with type 2 diabetes mellitus. J Diabetes Metab. 2011;7 doi:10.4172/2155-6156.1000142.
Shyangdan DS, Uthman OA, Waugh N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open. 2016;6:e009417. doi:10.1136/bmjopen-2015-009417.
Nyirjesy P, Sobel JD, Fung A, Mayer C, Capuano G, Ways K, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin. 2014;30:1109–19.
Usiskin K, Kline I, Fung A, Mayer C, Meininger G. Safety and tolerability of canagliflozin in patients with type 2 diabetes mellitus: pooled analysis of phase 3 study results. Postgrad Med. 2014;126:16–34.
http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm (FDA warning about SGLT-2i and DKA)
• Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransported 2 inhibition. Diab Care. 2015;38:1687–93. First report of SGLT-2 inhibitors association with diabetic ketoacidosis
Taylor SI, Blau JE, Mother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrine Metab. 2015;100:2849–52.
• Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22:753–62. International conference on association of SGLT-2 inhibitors and diabetic ketoacidosis reviewing both the underlying science and clinical implications
Fioretto P, Stefansson BV, Johnson E, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia. 2016;59:2026–39.
http://www.fda.gov/Drugs/DrugSafety/ucm500965.htm. (canagliflozin - foot amputation)
http://www.fda.gov/Safety/MedWatch/SafetyInformation SafetyAlertsforHumanMedicalProducts/ucm461876.htm. (canagliflozin - fractures)
http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202293s003lbl.pdf. (dapagligflozin—bladder cancer)
Wu JHY, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, et al. Effects of sodium-glucose contransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrine. 2016;4:411–9.
http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021332s006lbl.pdf. (pramlintide safety)
Grunberger G. Insulin analogs—are they worth it? Yes! Diab Care. 2014;37:1767–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
George Grunberger reports grants from Eli Lilly, Novo Nordisk, and AstraZeneca and speakers’ bureau fees from Eli Lilly, Novo Nordisk, Sanofi, Janssen, Boehringer Ingelheim, and AstraZeneca.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Grunberger, G. Should Side Effects Influence the Selection of Antidiabetic Therapies in Type 2 Diabetes?. Curr Diab Rep 17, 21 (2017). https://doi.org/10.1007/s11892-017-0853-8
Published:
DOI: https://doi.org/10.1007/s11892-017-0853-8