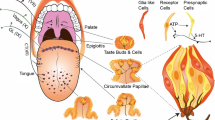
Abstract
The T1R2 (taste type 1 receptor, member 2)/T1R3 (taste type 1 receptor, member 3) sweet taste receptor is expressed in taste buds on the tongue, where it allows the detection of energy-rich carbohydrates of food. This single receptor responds to all compounds perceived as sweet by humans, including natural sugars and natural and artificial sweeteners. Importantly, the T1R2/T1R3 sweet taste receptor is also expressed in extra-oral tissues, including the stomach, pancreas, gut, liver, and brain. Although its physiological role remains to be established in numerous organs, T1R2/T1R3 is suspected to be involved in the regulation of metabolic processes, such as sugar sensing, glucose homeostasis, and satiety hormone release. In this review, the physiological role of the sweet taste receptor in taste perception and metabolic regulation is discussed by focusing on dysfunctions leading to diabetes. Current knowledge of T1R2/T1R3 inhibitors making this receptor a promising therapeutic target for the treatment of type 2 diabetes is also summarized and discussed.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99(7):4692–6. doi:10.1073/pnas.072090199.
Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106(3):381–90.
Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115(3):255–66.
Pin JP, Kniazeff J, Binet V, Liu J, Maurel D, Galvez T, et al. Activation mechanism of the heterodimeric GABA(B) receptor. Biochem Pharmacol. 2004;68(8):1565–72. doi:10.1016/j.bcp.2004.06.035.
Jiang P, Ji Q, Liu Z, Snyder LA, Benard LM, Margolskee RF, et al. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J Biol Chem. 2004;279(43):45068–75. doi:10.1074/jbc.M406779200.
Poirier N, Roudnitzky N, Brockhoff A, Belloir C, Maison M, Thomas-Danguin T, et al. Efficient production and characterization of the sweet-tasting brazzein secreted by the yeast Pichia pastoris. J Agric Food Chem. 2012;60(39):9807–14. doi:10.1021/jf301600m.
Ariyasu T, Matsumoto S, Kyono F, Hanaya T, Arai S, Ikeda M, et al. Taste receptor T1R3 is an essential molecule for the cellular recognition of the disaccharide trehalose. In Vitro Cell Dev Biol Anim. 2003;39(1-2):80–8. doi:10.1290/1543-706X(2003)039<0080:TRTIAE>2.0.CO;2.
Maitrepierre E, Sigoillot M, Le Pessot L, Briand L. Recombinant expression, in vitro refolding, and biophysical characterization of the N-terminal domain of T1R3 taste receptor. Protein Expr Purif. 2012;83(1):75–83. doi:10.1016/j.pep.2012.03.006.
Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol. 2005;15(21):1948–52. doi:10.1016/j.cub.2005.09.037.
Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301(5634):850–3. doi:10.1126/science.1087155.
Behrens M, Meyerhof W, Hellfritsch C, Hofmann T. Sweet and umami taste: natural products, their chemosensory targets, and beyond. Angew Chem Int Ed Engl. 2011;50(10):2220–42. doi:10.1002/anie.201002094.
Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci U S A. 2004;101(39):14258–63. doi:10.1073/pnas.0404384101.
Tizzano M, Dvoryanchikov G, Barrows JK, Kim S, Chaudhari N, Finger TE. Expression of Galpha14 in sweet-transducing taste cells of the posterior tongue. BMC Neurosci. 2008;9:110. doi:10.1186/1471-2202-9-110.
Eny KM, Wolever TM, Corey PN, El-Sohemy A. Genetic variation in TAS1R2 (Ile191Val) is associated with consumption of sugars in overweight and obese individuals in 2 distinct populations. Am J Clin Nutr. 2010;92(6):1501–10. doi:10.3945/ajcn.2010.29836.
Dias AG, Eny KM, Cockburn M, Chiu W, Nielsen DE, Duizer L, et al. Variation in the TAS1R2 gene, sweet taste perception and intake of sugars. J Nutrigenet Nutrigenomics. 2015;8(2):81–90. doi:10.1159/000430886.
Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr Biol. 2009;19(15):1288–93. doi:10.1016/j.cub.2009.06.015.
Fushan AA, Simons CT, Slack JP, Drayna D. Association between common variation in genes encoding sweet taste signaling components and human sucrose perception. Chem Senses. 2010;35(7):579–92. doi:10.1093/chemse/bjq063.
Gondivkar SM, Indurkar A, Degwekar S, Bhowate R. Evaluation of gustatory function in patients with diabetes mellitus type 2. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(6):876–80. doi:10.1016/j.tripleo.2009.08.015.
Wasalathanthri S, Hettiarachchi P, Prathapan S. Sweet taste sensitivity in pre-diabetics, diabetics and normoglycemic controls: a comparative cross sectional study. BMC Endocr Disord. 2014;14:67. doi:10.1186/1472-6823-14-67. The results presented in this paper confirmed a decrease in taste response for diabetics and showed a taste response for pre-diabetic as an intermediate between diabetic and normoglycemic patients.
Khobragade RS, Wakode SL, Kale AH. Physiological taste threshold in type 1 diabetes mellitus. Indian J Physiol Pharmacol. 2012;56(1):42–7.
Tepper BJ, Hartfiel LM, Schneider SH. Sweet taste and diet in type II diabetes. Physiol Behav. 1996;60(1):13–8.
Fabian TK, Beck A, Fejerdy P, Hermann P, Fabian G. Molecular mechanisms of taste recognition: considerations about the role of saliva. Int J Mol Sci. 2015;16(3):5945–74. doi:10.3390/ijms16035945.
Neyraud E. Role of saliva in oral food perception. Monogr Oral Sci. 2014;24:61–70. doi:10.1159/000358789.
Ben-Aryeh H, Cohen M, Kanter Y, Szargel R, Laufer D. Salivary composition in diabetic patients. J Diabet Complications. 1988;2(2):96–9.
Fabian TK, Fejerdy P, Csermely P. Salivary genomics, transcriptomics and proteomics: the emerging concept of the oral ecosystem and their use in the early diagnosis of cancer and other diseases. Curr Genomics. 2008;9(1):11–21. doi:10.2174/138920208783884900.
Koscielniak D, Jurczak A, Zygmunt A, Krzysciak W. Salivary proteins in health and disease. Acta Biochim Pol. 2012;59(4):451–7.
Falchi M, El-Sayed Moustafa JS, Takousis P, Pesce F, Bonnefond A, Andersson-Assarsson JC et al. Low copy number of the salivary amylase gene predisposes to obesity. Nat Genet. 2014;46(5):492-7. doi:10.1038/ng.2939. Using 6200 subjects, the authors identified an association between a low copy number variants of the salivary amylase gene ( AMY1 ) with high body mass index and obesity risk.
Mejia-Benitez MA, Bonnefond A, Yengo L, Huyvaert M, Dechaume A, Peralta-Romero J, et al. Beneficial effect of a high number of copies of salivary amylase AMY1 gene on obesity risk in Mexican children. Diabetologia. 2015;58(2):290–4. doi:10.1007/s00125-014-3441-3.
Indira M, Chandrashekar P, Kattappagari KK, Chandra LP, Chitturi RT, Bv RR. Evaluation of salivary glucose, amylase, and total protein in type 2 diabetes mellitus patients. Indian J Dent Res. 2015;26(3):271–5. doi:10.4103/0970-9290.162883.
Oxford GE, Tayari L, Barfoot MD, Peck AB, Tanaka Y, Humphreys-Beher MG. Salivary EGF levels reduced in diabetic patients. J Diabetes Complications. 2000;14(3):140–5.
Morris-Wiman J, Sego R, Brinkley L, Dolce C. The effects of sialoadenectomy and exogenous EGF on taste bud morphology and maintenance. Chem Senses. 2000;25(1):9–19.
Nagy A, Nagashima H, Cha S, Oxford GE, Zelles T, Peck AB, et al. Reduced oral wound healing in the NOD mouse model for type 1 autoimmune diabetes and its reversal by epidermal growth factor supplementation. Diabetes. 2001;50(9):2100–4.
Kerr M, Lee A, Wang PL, Purushotham KR, Chegini N, Yamamoto H, et al. Detection of insulin and insulin-like growth factors I and II in saliva and potential synthesis in the salivary glands of mice. Effects of type 1 diabetes mellitus. Biochem Pharmacol. 1995;49(10):1521–31.
Takai S, Yasumatsu K, Inoue M, Iwata S, Yoshida R, Shigemura N, et al. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015;29(6):2268–80. doi:10.1096/fj.14-265355.
Cai H, Maudsley S, Martin B. What is the role of metabolic hormones in taste buds of the tongue. Front Horm Res. 2014;42:134–46. doi:10.1159/000358322.
Calvo SS, Egan JM. The endocrinology of taste receptors. Nat Rev Endocrinol. 2015;11(4):213–27. doi:10.1038/nrendo.2015.7.
Kubasova N, Burdakov D, Domingos AI. Sweet and low on leptin: hormonal regulation of sweet taste buds. Diabetes. 2015;64(11):3651–2. doi:10.2337/dbi15-0004.
Yoshida R, Noguchi K, Shigemura N, Jyotaki M, Takahashi I, Margolskee RF et al. Leptin suppresses mouse taste cell responses to sweet compounds. Diabetes. 2015;64(11):3751-62. doi:10.2337/db14-1462. This paper shows that leptin selectively suppresses sweet taste perception, but not other perceptions, through the inhibition of K ATP channel co-expressed in the same taste cells.
Murovets VO, Bachmanov AA, Zolotarev VA. Impaired glucose metabolism in mice lacking the Tas1r3 taste receptor gene. PLoS One. 2015;10(6):e0130997. doi:10.1371/journal.pone.0130997.
Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK, et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58(3):337–46. doi:10.1136/gut.2008.148932.
Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32(1):41–9. doi:10.1093/chemse/bjl034.
Dyer J, Vayro S, King TP, Shirazi-Beechey SP. Glucose sensing in the intestinal epithelium. Eur J Biochem. 2003;270(16):3377–88.
Symonds EL, Peiris M, Page AJ, Chia B, Dogra H, Masding A, et al. Mechanisms of activation of mouse and human enteroendocrine cells by nutrients. Gut. 2015;64(4):618–26. doi:10.1136/gutjnl-2014-306834.
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104(38):15069–74. doi:10.1073/pnas.0706890104.
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104(38):15075–80. doi:10.1073/pnas.0706678104.
Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin Nutr. 2011;30(4):524–32. doi:10.1016/j.clnu.2011.01.007.
Shirazi-Beechey SP, Daly K, Al-Rammahi M, Moran AW, Bravo D. Role of nutrient-sensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br J Nutr. 2014;111 Suppl 1:S8–S15. doi:10.1017/S0007114513002286.
Young RL, Chia B, Isaacs NJ, Ma J, Khoo J, Wu T, et al. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes. 2013;62(10):3532–41. doi:10.2337/db13-0581. This study shows that intestinal T1R2 is less expressed in the presence of glucose for healthy subjects. In parallel, this downregulation is not observed in type 2 diabetes patients.
Swartz TD, Duca FA, de Wouters T, Sakar Y, Covasa M. Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br J Nutr. 2012;107(5):621–30. doi:10.1017/S0007114511003412.
Zhu X, He L, McCluskey LP. Ingestion of bacterial lipopolysaccharide inhibits peripheral taste responses to sucrose in mice. Neuroscience. 2014;258:47–61. doi:10.1016/j.neuroscience.2013.10.072.
Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. doi:10.1038/nature13793.
Nakagawa Y, Ohtsu Y, Nagasawa M, Shibata H, Kojima I. Glucose promotes its own metabolism by acting on the cell-surface glucose-sensing receptor T1R3. Endocr J. 2014;61(2):119–31.
Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A. 2012;109(8):E524–32. doi:10.1073/pnas.1115183109.
Malaisse WJ, Vanonderbergen A, Louchami K, Jijakli H, Malaisse-Lagae F. Effects of artificial sweeteners on insulin release and cationic fluxes in rat pancreatic islets. Cell Signal. 1998;10(10):727–33.
Kojima I, Nakagawa Y, Hamano K, Medina J, Li L, Nagasawa M. Glucose-sensing receptor T1R3: a new signaling receptor activated by glucose in pancreatic beta-cells. Biol Pharm Bull. 2015;38(5):674–9. doi:10.1248/bpb.b14-00895. This study demonstrates that in pancreatic cells, T1R3 acts as a glucose-sensing receptor, involved in glucose-induce insulin secretion.
Medina A, Nakagawa Y, Ma J, Li L, Hamano K, Akimoto T, et al. Expression of the glucose-sensing receptor T1R3 in pancreatic islet: changes in the expression levels in various nutritional and metabolic states. Endocr J. 2014;61(8):797–805. This paper highlights that T1R3 expression is modulated by the nutritional and metabolic states. A higher T1R3 expression is observed in beta cells in fasting condition.
Nakagawa Y, Nagasawa M, Mogami H, Lohse M, Ninomiya Y, Kojima I. Multimodal function of the sweet taste receptor expressed in pancreatic beta-cells: generation of diverse patterns of intracellular signals by sweet agonists. Endocr J. 2013;60(10):1191–206. This paper demonstrates that in pancreatic cells, the sweet taste receptor generates distinct patterns of intracellular signal following the sweetener used for its activation.
Kyriazis GA, Smith KR, Tyrberg B, Hussain T, Pratley RE. Sweet taste receptors regulate basal insulin secretion and contribute to compensatory insulin hypersecretion during the development of diabetes in male mice. Endocrinology. 2014;155(6):2112–21. doi:10.1210/en.2013-2015. This key study reveals that elevated glucose level reduces T1R2 expression in human pancreatic islet. Both pancreatic T1R2 and T1R3 present a lower expression level in obese or diabetic mouse model associated with an insulin hypersecretion. The insulin hypersecretion is also observed in T1R2 knockout mice in fasting condition.
Hardy ML, Coulter I, Venuturupalli S, Roth EA, Favreau J, Morton SC, et al. Ayurvedic interventions for diabetes mellitus: a systematic review. Evid Rep Technol Assess (Summ). 2001;41:2.
Galindo-Cuspinera V, Breslin PA. The liaison of sweet and savory. Chem Senses. 2006;31(3):221–5. doi:10.1093/chemse/bjj022.
Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LM, et al. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem. 2005;280(15):15238–46. doi:10.1074/jbc.M414287200.
Sanematsu K, Kusakabe Y, Shigemura N, Hirokawa T, Nakamura S, Imoto T, et al. Molecular mechanisms for sweet-suppressing effect of gymnemic acids. J Biol Chem. 2014;289(37):25711–20. doi:10.1074/jbc.M114.560409.
Sigoillot M, Brockhoff A, Meyerhof W, Briand L. Sweet-taste-suppressing compounds: current knowledge and perspectives of application. Appl Microbiol Biotechnol. 2012;96(3):619–30. doi:10.1007/s00253-012-4387-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Fabrice Neiers, Marie-Chantal Canivenc-Lavier, and Loïc Briand declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pathogenesis of Type 2 Diabetes and Insulin Resistance
Rights and permissions
About this article
Cite this article
Neiers, F., Canivenc-Lavier, MC. & Briand, L. What Does Diabetes “Taste” Like?. Curr Diab Rep 16, 49 (2016). https://doi.org/10.1007/s11892-016-0746-2
Published:
DOI: https://doi.org/10.1007/s11892-016-0746-2