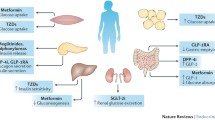
Abstract
Genetic variation can impact on efficacy and risk of adverse events to commonly used oral agents in diabetes. Metformin is not metabolized and its mechanism of action remains debated; however, several cation transporters have been identified. Variation in these pharmacokinetic genes might influence metformin response. Conversely, although the cytochrome P450 system has been implicated in sulfonylurea response in some small studies, to date variants affecting pharmacodynamics, including those in ABCC8 (SUR1) and TCF7L2, are the most promising. For thiazolidinedione response, variants in PPARG or ADIPOQ (adiponectin) have been variably associated with response. With increasing well-phenotyped cohorts and new methods, including genome-wide association studies, the next few years offer great hope to use pharmacogenetics to unravel drug and disease mechanisms, as well as the possibility to individualize therapy by genotype.
Similar content being viewed by others
References and Recommended Reading
Scott J, Poffenbarger PL: Pharmacogenetics of tolbutamide metabolism in humans. Diabetes 1979, 28:41–51.
Relling MV, Aoyama T, Gonzalez FJ, Meyer UA: Tolbutamide and mephenytoin hydroxylation by human cytochrome P450s in the CYP2C subfamily. J Pharmacol Exp Ther 1990, 252:442–447.
Kirchheiner J, Brockmoller J, Meineke I, et al.: Impact of CYP2C9 amino acid polymorphisms on glyburide kinetics and on the insulin and glucose response in healthy volunteers. Clin Pharmacol Ther 2002, 71:286–296.
Elliot DJ, Suharjono, Lewis BC, et al.: Identification of the human cytochromes P450 catalysing the rate-limiting pathways of gliclazide elimination. Br J Clin Pharmacol 2007, 64:450–457.
Kidd RS, Curry TB, Gallagher S, et al.: Identification of a null allele of CYP2C9 in an African-American exhibiting toxicity to phenytoin. Pharmacogenetics 2001, 11:803–808.
Wang R, Chen K, Wen SY, et al.: Pharmacokinetics of glimepiride and cytochrome P450 2C9 genetic polymorphisms. Clin Pharmacol Ther 2005, 78:90–92.
Kirchheiner J, Bauer S, Meineke I, et al.: Impact of CYP2C9 and CYP2C19 polymorphisms on tolbutamide kinetics and the insulin and glucose response in healthy volunteers. Pharmacogenetics 2002, 12:101–109.
Becker ML, Visser LE, Trienekens PH, et al.: Cytochrome P450 2C9 *2 and *3 polymorphisms and the dose and effect of sulfonylurea in type II diabetes mellitus. Clin Pharmacol Ther 2008, 83:288–292.
Zhang Y, Si D, Chen X, et al.: Influence of CYP2C9 and CYP2C19 genetic polymorphisms on pharmacokinetics of gliclazide MR in Chinese subjects. Br J Clin Pharmacol 2007, 64:67–74.
Zhou M, Xia L, Wang J: Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos 2007, 35:1956–1962.
Wang DS, Jonker JW, Kato Y, et al.: Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther 2002, 302:510–515.
Kimura N, Masuda S, Tanihara Y, et al.: Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet 2005, 20:379–386.
Wang DS, Kusuhara H, Kato Y, et al.: Involvement of organic cation transporter 1 in the lactic acidosis caused by metformin. Mol Pharmacol 2003, 63:844–848.
Shu Y, Sheardown SA, Brown C, et al.: Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 2007, 117:1422–1431.
Shu Y, Brown C, Castro RA, et al.: Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther 2008, 83:273–280.
Kirchheiner J, Thomas S, Bauer S, et al.: Pharmacokinetics and pharmacodynamics of rosiglitazone in relation to CYP2C8 genotype. Clin Pharmacol Ther 2006, 80:657–667.
Tornio A, Niemi M, Neuvonen PJ, Backman JT: Trimethoprim and the CYP2C8*3 allele have opposite effects on the pharmacokinetics of pioglitazone. Drug Metab Disp 2008, 36:73–80.
Pearson ER, Starkey BJ, Powell RJ, et al.: Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 2003, 362:1275–1281.
Shepherd M, Pearson ER, Houghton J, et al.: No deterioration in glycemic control in HNF-1alpha maturity-onset diabetes of the young following transfer from long-term insulin to sulphonylureas. Diabetes Care 2003, 26:3191–3192.
Gloyn AL, Pearson ER, Antcliff JF, et al.: Activating mutations in the gene encoding the ATP-sensitive potassiumchannel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 2004, 350:1838–1849.
Proks P, Arnold AL, Bruining J, et al.: A heterozygous activating mutation in the sulfphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet 2006, 15:1793–1800.
Babenko AP, Polak M, Cave H, et al.: Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med 2006, 355:456–466.
Sagen JV, Raeder H, Hathout E, et al.: Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes 2004, 53:2713–2718.
Pearson ER, Flechtner I, Njolstad PR, et al.: Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 2006, 355:467–477.
Frayling TM: Genome-wide association studies provide new insights into type 2 diabetes etiology. Nat Rev 2007, 8:657–662.
Feng Y, Mao G, Ren X, et al.: Ser1369Ala variant in sulfonylurea receptor gene ABCC8 is associated with antidiabetic efficacy of gliclazide in Chinese type 2 diabetic patients. Diabetes Care 2008, 31:1939–1944.
Gloyn AL, Weedon MN, Owen KR, et al.: Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 2003, 52:568–572.
Sesti G, Laratta E, Cardellini M, et al.: The E23K variant of KCNJ11 encoding the pancreatic beta-cell adenosine 5′-triphosphate-sensitive potassium channel subunit Kir6.2 is associated with an increased risk of secondary failure to sulfonylurea in patients with type 2 diabetes. J Clin Endocrinol Metab 2006, 91:2334–2339.
Gloyn AL, Hashim Y, Ashcroft SJ, et al.: Association studies of variants in promoter and coding regions of beta-cell ATP-sensitive K-channel genes SUR1 and Kir6.2 with type 2 diabetes mellitus (UKPDS 53). Diabet Med 2001, 18:206–212.
Grant SF, Thorleifsson G, Reynisdottir I, et al.: Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 2006, 38:320–323.
Saxena R, Gianniny L, Burtt NP, et al.: Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes 2006, 55:2890–2895.
Lyssenko V, Lupi R, Marchetti P, et al.: Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007, 117:2155–2163.
Florez JC, Jablonski KA, Bayley N, et al.: TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 2006, 355:241–250.
Pearson ER, Donnelly LA, Kimber C, et al.: Variation in TCF7L2 influences therapeutic response to sulfonylureas: a GoDARTs study. Diabetes 2007, 56:2178–2182.
Natali A, Ferrannini E: Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia 2006, 49:434–441.
Horikoshi M, Hara K, Ohashi J, et al.: A polymorphism in the AMPKalpha2 subunit gene is associated with insulin resistance and type 2 diabetes in the Japanese population. Diabetes 2006, 55:919–923.
Sun MW, Lee JY, de Bakker PI, et al.: Haplotype structures and large-scale association testing of the 5′ AMP-activated protein kinase genes PRKAA2, PRKAB1, and PRKAB2 [corrected] with type 2 diabetes. Diabetes 2006, 55:849–855.
Keshavarz P, Inoue H, Nakamura N, et al.: Single nucleotide polymorphisms in genes encoding LKB1 (STK11), TORC2 (CRTC2) and AMPK alpha2-subunit (PRKAA2) and risk of type 2 diabetes. Mol Genet Metab 2008, 93:200–209.
Legro RS, Barnhart HX, Schlaff WD, et al.: Ovulatory response to treatment of polycystic ovary syndrome is associated with a polymorphism in the STK11 gene. J Clin Endocrinol Metab 2008, 93:792–800.
Yki-Jarvinen H: Thiazolidinediones. N Engl J Med 2004, 351:1106–1118.
Altshuler D, Hirschhorn JN, Klannemark M, et al.: The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 2000, 26:76–80.
Muller YL, Bogardus C, Beamer BA, et al.: A functional variant in the peroxisome proliferator-activated receptor gamma2 promoter is associated with predictors of obesity and type 2 diabetes in Pima Indians. Diabetes 2003, 52:1864–1871.
Bluher M, Lubben G, Paschke R: Analysis of the relationship between the Pro12Ala variant in the PPAR-gamma2 gene and the response rate to therapy with pioglitazone in patients with type 2 diabetes. Diabetes Care 2003, 26:825–831.
Snitker S, Watanabe RM, Ani I, et al.: Changes in insulin sensitivity in response to troglitazone do not differ between subjects with and without the common, functional Pro12Ala peroxisome proliferator-activated receptor-gamma2 gene variant: results from the Troglitazone in Prevention of Diabetes (TRIPOD) study. Diabetes Care 2004, 27:1365–1368.
Wolford JK, Yeatts KA, Dhanjal SK, et al.: Sequence variation in PPARG may underlie differential response to troglitazone. Diabetes 2005, 54:3319–3325.
Florez JC, Jablonski KA, Sun MW, et al.: Effects of the type 2 diabetes-associated PPARG P12A polymorphism on progression to diabetes and response to troglitazone. J Clin Endocrinol Metab 2007, 92:1502–1509.
Kang ES, Park SY, Kim HJ, et al.: Effects of Pro12Ala polymorphism of peroxisome proliferator-activated receptor gamma2 gene on rosiglitazone response in type 2 diabetes. Clin Pharmacol Ther 2005, 78:202–208.
Kang ES, Park SY, Kim HJ, et al.: The influence of adiponectin gene polymorphism on the rosiglitazone response in patients with type 2 diabetes. Diabetes Care 2005, 28:1139–1144.
Sun H, Gong ZC, Yin JY, et al.: The association of adiponectin allele 45T/G and -11377C/G polymorphisms with Type 2 diabetes and rosiglitazone response in Chinese patients. Br J Clin Pharmacol 2008, 65:917–926.
Brunham LR, Kruit JK, Pape TD, et al.: Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 2007, 13:340–347.
Wang J, Bao YQ, Hu C, et al.: Effects of ABCA1 variants on rosiglitazone monotherapy in newly diagnosed type 2 diabetes patients. Acta Pharmacol Sin 2008, 29:252–258.
Spraggs C, McCarthy A, McCarthy L, et al.: Genetic variants in the epithelial sodium channel associate with oedema in type 2 diabetic patients receiving the peroxisome proliferator-activated receptor gamma agonist farglitazar. Pharmacogenet Genomics 2007, 17:1065–1076.
Hansen L, Ekstrom CT, Tabanera YPR, et al.: The Pro12Ala variant of the PPARG gene is a risk factor for peroxisome proliferator-activated receptor-gamma/alpha agonist-induced edema in type 2 diabetic patients. J Clin Endocrinol Metab 2006, 91:3446–3450.
Link E, Parish S, Armitage J, et al.: SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008, 359:789–799.
Holstein A, Plaschke A, Ptak M, et al.: Association between CYP2C9 slow metabolizer genotypes and severe hypoglycaemia on medication with sulphonylurea hypoglycaemic agents. Br J Clin Pharmacol 2005, 60:103–106.
Sesti G, Marini MA, Cardellini M, et al.: The Arg972 variant in insulin receptor substrate-1 is associated with an increased risk of secondary failure to sulfonylurea in patients with type 2 diabetes. Diabetes Care 2004, 27:1394–1398.
Kang ES, Cha BS, Kim HJ, et al.: The 11482G >A polymorphism in the perilipin gene is associated with weight gain with rosiglitazone treatment in type 2 diabetes. Diabetes Care 2006, 29:1320–1324.
Wang G, Wang X, Zhang Q, Ma Z: Response to pioglitazone treatment is associated with the lipoprotein lipase S447X variant in subjects with type 2 diabetes mellitus. Int J Clin Pract 2007, 61:552–557.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pearson, E.R. Pharmacogenetics in diabetes. Curr Diab Rep 9, 172–181 (2009). https://doi.org/10.1007/s11892-009-0028-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-009-0028-3