Abstract
Insulin resistance plays a major role in the pathogenesis of the metabolic syndrome and type 2 diabetes, and yet the mechanisms responsible for it remain poorly understood. Magnetic resonance spectroscopy studies in humans suggest that a defect in insulin-stimulated glucose transport in skeletal muscle is the primary metabolic abnormality in insulin-resistant patients with type 2 diabetes. Fatty acids appear to cause this defect in glucose transport by inhibiting insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1) and IRS-1-associated phosphatidylinositol 3-kinase activity. A number of different metabolic abnormalities may increase intramyocellular and intrahepatic fatty acid metabolites; these include increased fat delivery to muscle and liver as a consequence of either excess energy intake or defects in adipocyte fat metabolism, and acquired or inherited defects in mitochondrial fatty acid oxidation. Understanding the molecular and biochemical defects responsible for insulin resistance is beginning to unveil novel therapeutic targets for the treatment of the metabolic syndrome and type 2 diabetes.
Similar content being viewed by others
References and Recommended Reading
Zimmet P, Alberti KG, Shaw J: Global and societal implications of the diabetes epidemic. Nature 2001, 414:782–787.
Lillioja S, Mott DM, Howard BV, et al.: Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med 1988, 318:1217–1225.
Lillioja S, Mott DM, Spraul M, et al.: Insulin resistance and insulin secretory dysfunction as precursors of non-insulindependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993, 329:1988–1992.
DeFronzoRA: Pathogenesis of type 2 (non-insulin dependent) diabetes mellitus: a balanced overview. Diabetologia 1992, 35:389–397.
Warram JH, Martin BC, Krolewski AS, et al.: Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 1990, 113:909–915.
Azen SP, Peters RK, Berkowitz K, et al.: TRIPOD (TRoglitazone In the Prevention Of Diabetes): a randomized, placebocontrolled trial of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials 1998, 19:217–231.
Shulman GI, Rothman DL, Jue T, et al.: Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 1990, 322:223–228.
Rothman DL, Shulman RG, Shulman GI: 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest 1992, 89:1069–1075.
Rothman DL, Magnusson I, Cline G, et al.: Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A 1995, 92:983–987.
Cline GW, Petersen KF, Krssak M, et al.: Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med 1999, 341:240–246.
Boden G, Shulman GI: Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002, 32(suppl 3):14–23.
Krssak M, Petersen KF, Dresner A, et al.: Intramyocellular lipid concentrations are correlated with insulin sensitivity in man: a 1H NMR spectroscopy study. Diabetologia 1999, 41:113–116.
Roden M, Price TB, Perseghin G, et al.: Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 1996, 97:2859–2865.
Petersen KF, Hendler R, Price T, et al.: 13C/31P NMR studies on the mechanism of insulin resistance in obesity. Diabetes 1998, 47:381–386.
Dresner A, Laurent D, Marcucci M, et al.: Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 1999, 103:253–259.
Griffin ME, Marcucci MJ, Cline GW, et al.: Free fatty acidinduced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 1999, 48:1270–1274.
Yin MJ, Yamamoto Y, Gaynor RB: The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 1998, 396:77–80.
Yuan M, Konstantopoulos N, Lee J, et al.: Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001, 293:1673–1677.
Kim JK, Kim YJ, Fillmore JJ, et al.: Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 2001, 108:437–446.
Hundal RS, Petersen KF, Mayerson AB, et al.: Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest 2002, 109:1321–1326.
Yu C, Chen Y, Cline GW, et al.: Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 2002, 277:50230–50236.
Itani SI, Ruderman NB, Schmieder F, Boden G: Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaBalpha. Diabetes 2002, 51:2005–2011.
Hotamisligil GS, Peraldi P, Budavari A, et al.: IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271:665–668.
Bjorntorp P: Abdominal fat distribution and the metabolic syndrome. J Cardiovasc Pharmacol 1992, 20(suppl 8):S26-S28.
Foley JE, Thuillez P, Lillioja S, et al.: Insulin sensitivity in adipocytes from subjects with varying degrees of glucose tolerance. Am J Physiol 1986, 251:E306-E310.
Saghizadeh M, Ong JM, Garvey WT, et al.: The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest 1996, 97:1111–1116.
Beisel WR: Herman Award Lecture, 1995: infection-induced malnutrition-from cholera to cytokines. Am J Clin Nutr 1995, 62:813–819.
Steppan CM, Lazar MA: Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab 2002, 13:18–23.
Fukuhara A, Matsuda M, Nishizawa M, et al.: Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2005, 307:426–430.
Hug C, Lodish HF: Medicine. Visfatin: a new adipokine. Science 2005, 307:366–367.
Moitra J, Mason MM, Olive M, et al.: Life without white fat: a transgenic mouse. Genes Dev 1998, 12:3168–3181.
Gavrilova O, Marcus-Samuels B, Graham D, et al.: Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 2000, 105:271–278.
Kim JK, Gavrilova O, Chen Y, et al.: Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem 2000, 275:8456–8460.
Petersen KF, Oral EA, Dufour S, et al.: Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 2002, 109:1345–1350. Showed that patients with generalized lipodystrophy had severe type 2 diabetes, hepatic steatosis, and hepatic insulin resistance in the absence of intra-abdominal fat. During leptin replacement treatment, the hepatic lipid stores were mobilized and this was associated with normalization of hepatic glucose production and suppression by insulin. The study strongly supports the hypothesis that hepatic lipid accumulation causes hepatic insulin resistance.
Conley KE, Jubrias SA, Esselman PC: Oxidative capacity and ageing in human muscle. J Physiol 2000, 526(Pt 1):203–210.
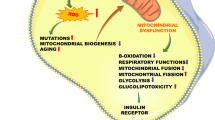
Petersen KF, Befroy D, Dufour S, et al.: Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003, 300:1140–1142. The insulin clamp and 13C and 31P MRS techniques were used to assess muscle insulin sensitivity and mitochondrial oxidative function in lean, sedentary elderly subjects. The findings support the hypothesis that an age-associated decline in mitochondrial function contributes to insulin resistance in the elderly by an age-associated accumulation of IMCL.
Michikawa Y, Mazzucchelli F, Bresolin N, et al.: Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science 1999, 286:774–779.
Petersen KF, Dufour S, Befroy D, et al.: Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004, 350:664–671. Illustrated that insulin resistance in lean offspring of parents with type 2 diabetes is associated with dysregulation of intramyocellular fatty acid metabolism, possibly due to an inherited defect in mitochondrial oxidative function.
Wu Z, Puigserver P, Andersson U, et al.: Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98:115–124.
Lin J, Wu H, Tarr PT, et al.: Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002, 418:797–801.
Zong H, Ren JM, Young LH, et al.: AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A 2002, 99:15983–15987.
Wu H, Kanatous SB, Thurmond FA, et al.: Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 2002, 296:349–352.
Patti ME, Butte AJ, Crunkhorn S, et al.: Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 2003, 100:8466–8471.
Mikines KJ, Sonne B, Farrell PA, et al.: Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol 1988, 254:E248-E259.
Perseghin G, Price TB, Petersen KF, et al.: Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 1996, 335:1357–1362.
Knowler WC, Barrett-Connor E, Fowler SE, et al.: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002, 346:393–403.
Henry RR, Scheaffer L, Olefsky JM: Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1985, 61:917–925.
Petersen KF, Dufour S, Befroy D, et al.: Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance and hyperglycemia by moderate weight reduction in patients with type 2 diabetes mellitus. Diabetes 2005, 54:603–608.
Jeng CY, Sheu WH, Fuh MM, et al.: Relationship between hepatic glucose production and fasting plasma glucose concentration in patients with NIDDM. Diabetes 1994, 43:1440–1444.
Maggs DG, Buchanan TA, Burant CF, et al.: Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1998, 128:176–185.
Magnusson I, Rothman DL, Katz LD, et al.: Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 1992, 90:1323–1327.
Hundal RS, Krssak M, Dufour S, et al.: Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49:2063–2069.
Landau BR, Wahren J, Chandramouli V, et al.: Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 1996, 98:378–385.
Petersen KF, Krssak M, Navarro V, et al.: Contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in cirrhosis. Am J Physiol 1999, 276:E529-E535.
Mayerson AB, Hundal RS, Dufour S, et al.: The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 2002, 51:797–802. Demonstrated that thiazolidinedione therapy enhances insulin sensitivity in patients with type 2 diabetes by promoting increased insulin sensitivity in peripheral adipocytes. The results support the hypothesis that a redistribution of fat from insulin-responsive organs into peripheral adipocytes will improve insulin sensitivity.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parish, R., Petersen, K.F. Mitochondrial dysfunction and type 2 diabetes. Curr Diab Rep 5, 177–183 (2005). https://doi.org/10.1007/s11892-005-0006-3
Issue Date:
DOI: https://doi.org/10.1007/s11892-005-0006-3