Abstract
Purpose of Review
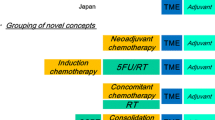
The currently established standard of care treatment for locally advanced rectal cancer (LARC) is neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision (TME) and adjuvant fluorouracil and oxaliplatin. The body of evidence that supports this treatment has grown over the last 20 years. However, recent advances and ongoing studies seek to further evaluate the role of neoadjuvant chemotherapy with and without radiation and total neoadjuvant therapy (TNT). In this article, we review the current literature as well as investigate the emerging role of TNT for patients with LARC and comment on updates utilizing combination neoadjuvant chemotherapy in early-stage rectal cancer.
Recent Findings
Evidence for the current standard of neoadjuvant CRT comes from well-established randomized phase III trials as well as emerging evidence on merits of TNT. There is a growing body of literature including retrospective analysis and ongoing clinical trials that look at upfront induction chemotherapy in addition to CRT prior to surgical resection leading to more effective delivery of systemic therapy and increases in response to treatment. Neoadjuvant combination chemotherapy is also being investigated in early-stage low rectal tumors to see if rates of local excision will increase compared to radical excision.
Summary
Current evidence continues to support neoadjuvant CRT as the standard treatment for LARC. There is an increasing body of evidence to support TNT in LARC as an effective treatment strategy that better ensures delivery of systemic therapy leading to higher rates of complete response (CR) and is encouraging for the development of non-operative protocols. There is ongoing evaluation looking at the benefit of novel sensitizers added to neoadjuvant therapy and ongoing investigation into upfront combination chemotherapy with selective use of radiation in upper rectal cancers.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
American Cancer Society Annual Cancer Facts and Figures. 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed 4 Mar 2020.
Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu A, et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19:2822–32.
Benson AB, Al-Hawary MM, Arain MA, et al. 2020. NCCN Guidelines Version 2.2020 Rectal Cancer Continue NCCN Guidelines Panel Disclosures. Accessed 3 Mar 2020.
Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4:e180071 A retrospective cohort analysis revealing higher rates of completion of planned treatment in patients undergoing TNT versus traditional adjuvant chemotherapy.
Peeters KCMJ, Marijnen CAM, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701.
Tekkis PP, Heriot AG, Smith J, Thompson MR, Finan P, Stamatakis JD. Comparison of circumferential margin involvement between restrorative and nonrestorative resections for rectal cancer. Color Dis. 2005;7:369–74.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Leichman CG, McDonough SL, Smalley SR, et al. Cetuximab combined with induction oxaliplatin and capecitabine, followed by neoadjuvant chemoradiation for locally advanced rectal cancer: SWOG 0713. Clin Colorectal Cancer. 2018;17:e121–5 Phase II study showing the addition of EGFR-targeted antibody cetuximab to neoadjuvant CRT in KRAS wild-type patients does not improve pCR.
Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase ii clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). 2012. https://doi.org/10.1200/JCO.2011.39.6036.
Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet. 2010;375:1030–47.
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33.
Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579–88.
Schrag D, Weiser MR, Goodman KA, Gon̈en M, Hollywood E, Cercek A, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–8.
Sainato A, Cernusco Luna Nunzia V, Valentini V, de Paoli A, Maurizi ER, Lupattelli M, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113:223–9.
Breugom AJ, van Gijn W, Muller EW, Berglund Å, van den Broek CBM, Fokstuen T, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26:696–701.
Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25:1356–62.
Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–90.
Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–28.
George TJ, Allegra CJ, Yothers G. Neoadjuvant rectal (NAR) score: a new surrogate endpoint in rectal cancer clinical trials. Curr Colorectal Cancer Rep. 2015;11:275–80.
Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–80.
Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Lafay I, Hennequin C, Etienne PL, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30:4558–65.
Schmoll H-J, Haustermans K, Price TJ, Nordlinger B, Hofheinz R, Daisne JF, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: disease-free survival results at interim analysis. J Clin Oncol. 2014;32:3501–3501.
Allegra CJ, Yothers G, O’Connell MJ, Beart RW, Wozniak TF, Pitot HC, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107:djv248. https://doi.org/10.1093/jnci/djv248.
Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34:3300–7.
O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from national surgical adjuvant breast and bowel project trial R-04. J Clin Oncol. 2014;32:1927–34.
Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–89.
Gollins S, Sun Myint A, Haylock B, Wise M, Saunders M, Neupane R, et al. Preoperative chemoradiotherapy using concurrent capecitabine and irinotecan in magnetic resonance imaging-defined locally advanced rectal cancer: impact on long-term clinical outcomes. J Clin Oncol. 2011;29:1042–9.
Navarro M, Dotor E, Rivera F, Sánchez-Rovira P, Vega-Villegas ME, Cervantes A, et al. A phase II study of preoperative radiotherapy and concomitant weekly irinotecan in combination with protracted venous infusion 5-fluorouracil, for resectable locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:201–5.
Mohiuddin M, Paulus R, Mitchell E, Hanna N, Yuen A, Nichols R, et al. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Int J Radiat Oncol Biol Phys. 2013;86:523–8.
Sebag-Montefiore DA. Phase III trial comparing standard versus novel CRT as pre-operative treatment for MRI defined locally advanced rectal cancer. http://www.ctc.ucl.ac.uk/TrialDetails.aspx?Trial=82&TherA=7. Accessed 27 Mar 2020. A phase III randomized controlled trial that has finished accrual investigating irinotecan as a radiosensitizer directly compared to standard CRT.
Mengual-Ballester M, García-Marín JA, Pellicer-Franco E, Guillén-Paredes MP, García-García ML, Cases-Baldó MJ, et al. Ileostomías de protección: complicaciones y mortalidad asociadas a su cierre. Rev Esp Enferm Dig. 2012;104:350–4.
Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668–74.
Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo Cáncer de Recto 3 study. J Clin Oncol. 2010;28:859–65.
Sclafani F, Peckitt C, Cunningham D, Evans J, Brown G, Tabernero J, et al. Panex: a pooled analysis of EXPERT and EXPERT-C, two trials of neoadjuvant chemotherapy (NACT) and chemoradiotherapy (CRT) in high-risk locally advanced rectal cancer (LARC). J Clin Oncol. 2014;32:3575–3575.
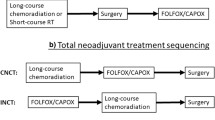
Conroy T, Lamfichekh N, Etienne P-L, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol. 2020;38:4007–4007 Investigated and found compliance to CRT was not negatively impacted by neoadjuvant chemotherapy and that TNT significantly increased ypCR rate as well as 3-year DFS compared to traditional CRT and adjuvant chemotherapy.
van Dijk TH, Tamas K, Beukema JC, Beets GL, Gelderblom AJ, de Jong KP, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol. 2013;24:1762–9.
Hospers G, Bahadoer RR, Dijkstra EA, et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: the randomized RAPIDO trial. J Clin Oncol. 2020;38:4006–4006 An investigation of short-course radiotherapy followed by chemotherapy prior to TME compared to standard CRT. Results with significantly lower disease-related treatment failure rate in high-risk LARC patients.
Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, Joyce M, et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25:4850–69.
Deng Y, Chi P, Lan P, et al. Neoadjuvant modified folfox6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial. J Clin Oncol. 2019;37:3223–33 A phase III prospective study showing no difference in 3-year DFS and OS and less treatment-related toxicity with neoadjuvant chemotherapy alone compared to CRT.
Havenga K, Enker WE, Norstein J, Moriya Y, Heald RJ, van Houwelingen HC, et al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol. 1999;25:368–74.
Bosset J-F, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23.
Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–5.
van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–45.
Garcia-Aguilar J, Patil S, Kim JK, Yuval JB, Thompson H, Verheij F, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 2020;38:4008–4008 Prospective study that showed comparable DFS between induction and consolidation chemotherapy + CRT in addition to high organ preservation rate in the consolidation arm allowing adoption of a watch and wait approach in patients who achieve clinical complete response with TNT.
Marijnen CAM, Kapiteijn E, van de Velde CJH, Martijn H, Steup WH, Wiggers T, et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2002;20:817–25.
Hendren SK, O’Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005;242:212–23.
Cercek A, Goodman KA, Hajj C, Weisberger E, Segal NH, Reidy-Lagunes DL, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. JNCCN J Natl Compr Cancer Netw. 2014;12:513–9.
Verseveld M, de Graaf EJR, Verhoef C, van Meerten E, Punt CJA, de Hingh IHJT, et al. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg. 2015;102:853–60.
Garcia-Aguilar J, Shi Q, Thomas CR, Chan E, Cataldo P, Marcet J, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384–91.
Pucciarelli S, de Paoli A, Guerrieri M, la Torre G, Maretto I, de Marchi F, et al. Local excision after preoperative chemoradiotherapy for rectal cancer: results of a multicenter phase II clinical trial. Dis Colon Rectum. 2013;56:1349–56.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Namrata Vijayvergia has received consultancy fees from Lexicon, Novartis, HalioDx, and Sun Pharma and grants from Merck and Bayer. Thomas Holden declares that he has no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Systemic Therapies in Colorectal Cancer
Rights and permissions
About this article
Cite this article
Holden, T., Vijayvergia, N. Review and Updates on Approaches to Neoadjuvant Chemotherapy in Rectal Cancer. Curr Colorectal Cancer Rep 17, 1–9 (2021). https://doi.org/10.1007/s11888-020-00462-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-020-00462-3