Abstract
Purpose of Review
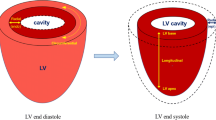
Heart failure results in the high incidence and mortality all over the world. Mechanical properties of myocardium are critical determinants of cardiac function, with regional variations in myocardial contractility demonstrated within infarcted ventricles. Quantitative assessment of cardiac contractile function is therefore critical to identify myocardial infarction for the early diagnosis and therapeutic intervention.
Recent Findings
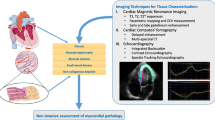
Current advancement of cardiac functional assessments is in pace with the development of imaging techniques. The methods tailored to advanced imaging have been widely used in cardiac magnetic resonance, echocardiography, and optical microscopy. In this review, we introduce fundamental concepts and applications of representative methods for each imaging modality used in both fundamental research and clinical investigations. All these methods have been designed or developed to quantify time-dependent 2-dimensional (2D) or 3D cardiac mechanics, holding great potential to unravel global or regional myocardial deformation and contractile function from end-systole to end-diastole.
Summary
Computational methods to assess cardiac contractile function provide a quantitative insight into the analysis of myocardial mechanics during cardiac development, injury, and remodeling.





Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Mann DL. Inflammatory mediators and the failing heart. Circ Res. 2002;91(11). https://doi.org/10.1161/01.RES.0000043825.01705.1B.
Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17(5). https://doi.org/10.1038/s41569-019-0315-x.
Jafar TH, et al. Non-communicable diseases and injuries in Pakistan: strategic priorities. The Lancet. 2013;381(9885). https://doi.org/10.1016/S0140-6736(13)60646-7.
Ponikowski P, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;(1). https://doi.org/10.1002/ehf2.12005.
Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1). https://doi.org/10.1038/nrcardio.2010.165.
Voorhees AP, Han H-C. Biomechanics of cardiac function. Compr Physiol. 2015;5(4). https://doi.org/10.1002/cphy.c140070.
Wang H, Amini AA. Cardiac motion and deformation recovery from MRI: a review. IEEE Trans Med Imaging. 2012;31(2). https://doi.org/10.1109/TMI.2011.2171706.
Frangi AF, Niessen WJ, Viergever MA. Three-dimensional modeling for functional analysis of cardiac images, a review. IEEE Trans Med Imaging 2001;20(1). https://doi.org/10.1109/42.906421.
Konstam MA, Abboud FM. Ejection fraction: misunderstood and overrated (changing the paradigm in categorizing heart failure). Circulation 2017;135(8). https://doi.org/10.1161/CIRCULATIONAHA.116.025795.
• Amzulescu MS, et al. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging. 2019;20(6). https://doi.org/10.1093/ehjci/jez041. This study compares specific cardiac tracking and strain imaging modalities, and it summarizes the general principles and technical innovations of current deformable image analysis.
Mirsky I, Parmley WW. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ Res. 1973;33(2):233–43. https://doi.org/10.1161/01.RES.33.2.233.
Abraham TP, Nishimura RA. Myocardial strain: can we finally measure contractility? J Am Coll Cardiol. 2001;37(3). https://doi.org/10.1016/s0735-1097(00)01173-6.
Sonnenblick EH. Instantaneous force-velocity-length determinants in the contraction of heart muscle. Circ Res. 1965;16(5). https://doi.org/10.1161/01.RES.16.5.441.
Scatteia A, Baritussio A, Bucciarelli-Ducci C. Strain imaging using cardiac magnetic resonance. Heart Fail Rev. 2017;22(4). https://doi.org/10.1007/s10741-017-9621-8.
Slomka P, Berman DS, Alexanderson E, Germano G. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging. 2014;2(4):343–58. https://doi.org/10.1007/s40336-014-0070-2.
Vach M, et al. Feasibility of CT-derived myocardial strain measurement in patients with advanced cardiac valve disease. Sci Rep. 2021;11(1). https://doi.org/10.1038/s41598-021-88294-5.
O’Dell WG, McVeigh ER, Moore CC, Zerhouni EA. Implementation of displacement field fitting for calculating 3D myocardial deformations from parallel-tagged MR images. Proc 16th Ann Int Conf IEEE Eng Med Biol Soc. 1994;1:551–552. https://doi.org/10.1109/IEMBS.1994.411908.
Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology 1989;171(3). https://doi.org/10.1148/radiology.171.3.2717762.
Ibrahim E-SH. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques–pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson. 2011;13(1). https://doi.org/10.1186/1532-429X-13-36.
Brower RW, ten Katen HJ, Meester GT. Direct method for determining regional myocardial shortening after bypass surgery from radiopaque markers in man. Am J Cardiol. 1978;41(7). https://doi.org/10.1016/0002-9149(78)90879-2.
Villarreal FJ, Waldman LK, Lew WY. Technique for measuring regional two-dimensional finite strains in canine left ventricle. Circ Res.1988;62(4). https://doi.org/10.1161/01.RES.62.4.711.
Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11(1). https://doi.org/10.1186/1532-429X-11-55.
Fischer SE, McKinnon GC, Maier SE, Boesiger P. Improved myocardial tagging contrast. Magn Reson Med. 1993;30(2). https://doi.org/10.1002/mrm.1910300207.
Aletras AH, Ding S, Balaban RS, Wen H. DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson San Diego Calif 1997. 1999;137(1). https://doi.org/10.1006/jmre.1998.1676.
Kim D, Epstein FH, Gilson WD, Axel L. Increasing the signal-to-noise ratio in DENSE MRI by combining displacement-encoded echoes. Magn Reson Med. 2004;52(1). https://doi.org/10.1002/mrm.20109.
Kim D, Gilson WD, Kramer CM, Epstein FH. Myocardial tissue tracking with two-dimensional cine displacement-encoded mr imaging: development and initial evaluation. Radiology 2004;230(3). https://doi.org/10.1148/radiol.2303021213.
Hess AT, Zhong X, Spottiswoode BS, Epstein FH, Meintjes EM. Myocardial 3D strain calculation by combining cine DENSE and cine SENC imaging. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2009;62(1). https://doi.org/10.1002/mrm.21984.
Guttman MA, Prince JL, McVeigh ER. Tag and contour detection in tagged MR images of the left ventricle. IEEE Trans Med Imaging 1994;13(1). https://doi.org/10.1109/42.276146.
Kumar S, Goldgof D. Automatic tracking of SPAMM grid and the estimation of deformation parameters from cardiac MR images. IEEE Trans Med Imaging 1994;13(1). https://doi.org/10.1109/42.276150.
Ozturk C, McVeigh ER. Four-dimensional B-spline based motion analysis of tagged MR images: introduction andin vivovalidation. Phys Med Biol. 2000;45(6). https://doi.org/10.1088/0031-9155/45/6/319.
Dougherty L, Asmuth JC, Blom AS, Axel L, Kumar R. Validation of an optical flow method for tag displacement estimation. IEEE Trans Med Imaging 1999;18(4). https://doi.org/10.1109/42.768845.
Florack L, van Assen H. A new methodology for multiscale myocardial deformation and strain analysis based on tagging MRI. Int J Biomed Imaging 2010:341242. https://doi.org/10.1155/2010/341242.
Xu C, et al. Deformation analysis of 3D tagged cardiac images using an optical flow method. J Cardiovasc Magn Reson. 2010;12(1). https://doi.org/10.1186/1532-429X-12-19.
Young AA. Model tags: direct three-dimensional tracking of heart wall motion from tagged magnetic resonance images. Med Image Anal. 1999;3(4). https://doi.org/10.1016/s1361-8415(99)80029-2.
Genet M, Stoeck CT, von Deuster C, Lee LC, Kozerke S. Equilibrated warping: finite element image registration with finite strain equilibrium gap regularization. Med Image Anal. 2018;50:1–22. https://doi.org/10.1016/j.media.2018.07.007.
Deng X, Denney TS. Combined tag tracking and strain reconstruction from tagged cardiac MR images without user-defined myocardial contours. J Magn Reson Imaging JMRI. 2005;21(1). https://doi.org/10.1002/jmri.20234.
Park J, Metaxas D, Axel L. Volumetric deformable models with parameter functions: A new approach to the 3D motion analysis of the LV from MRI-SPAMM. Proc IEEE Int Conf Comput Vision 1995:700–705. https://doi.org/10.1109/ICCV.1995.466870.
Sermesant M, Delingette H, Ayache N. An electromechanical model of the heart for image analysis and simulation. IEEE Trans Med Imaging 2006;25(5). https://doi.org/10.1109/TMI.2006.872746.
Osman NF, Kerwin WS, McVeigh ER, Prince JL. Cardiac motion tracking using CINE harmonic phase (HARP) magnetic resonance imaging. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 1999;42(6).
Osman NF, McVeigh ER, Prince JL. Imaging heart motion using harmonic phase MRI. IEEE Trans Med Imaging 2000;19(3). https://doi.org/10.1109/42.845177.
Osman NF, Prince JL. Visualizing myocardial function using HARP MRI. Phys Med Biol. 2000;45(6). https://doi.org/10.1088/0031-9155/45/6/318.
Arts T, Prinzen FW, Delhaas T, Milles JR, Rossi AC, Clarysse P. Mapping displacement and deformation of the heart with local sine-wave modeling. IEEE Trans Med Imaging. 2010;29(5):1114–23. https://doi.org/10.1109/TMI.2009.2037955.
Wang H, Stoeck CT, Kozerke S, Amini AA. Analysis of 3D cardiac deformations with 3D SinMod. Ann Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Ann Int Conf. 2013;2013:4386–9. https://doi.org/10.1109/EMBC.2013.6610518.
Clarysse P, et al. Two-dimensional spatial and temporal displacement and deformation field fitting from cardiac magnetic resonance tagging. Med Image Anal. 2000;4(3):253–68. https://doi.org/10.1016/s1361-8415(00)00018-9.
Ibrahim E-SH, et al. Regional cardiac function analysis from tagged MRI images. Comparison of techniques: harmonic-phase (HARP) versus sinusoidal-modeling (SinMod) analysis. Magn Reson Imaging 2018;54:271–282. https://doi.org/10.1016/j.mri.2018.05.008.
Kadappu KK, Thomas L. Tissue Doppler imaging in echocardiography: value and limitations. Heart Lung Circ. 2015;24(3). https://doi.org/10.1016/j.hlc.2014.10.003.
Isaaz K, Thompson A, Ethevenot G, Cloez JL, Brembilla B, Pernot C. Doppler echocardiographic measurement of low velocity motion of the left ventricular posterior wall. Am J Cardiol. 1989;64(1). https://doi.org/10.1016/0002-9149(89)90655-3.
Olsen NT, Jons C, Fritz-Hansen T, Mogelvang R, Sogaard P. Pulsed-wave tissue Doppler and color tissue doppler echocardiography: calibration with M-mode, agreement, and reproducibility in a clinical setting. Echocardiography. 2009;26(6):638–44. https://doi.org/10.1111/j.1540-8175.2008.00872.x.
Soliman OII, et al. Spectral pulsed-wave tissue Doppler imaging lateral-to-septal delay fails to predict clinical or echocardiographic outcome after cardiac resynchronization therapy. EP Eur. 2007;9(2):113–8. https://doi.org/10.1093/europace/eul149.
Sahn DJ. Instrumentation and physical factors related to visualization of stenotic and regurgitant jets by Doppler color flow mapping. J Am Coll Cardiol. 1988;12(5):1354–65. https://doi.org/10.1016/0735-1097(88)92621-6.
Nestaas E, Schubert U, de Boode WP, El-Khuffash A. Tissue Doppler velocity imaging and event timings in neonates: a guide to image acquisition, measurement, interpretation, and reference values. Pediatr Res. 2018;84(Suppl 1):18–29. https://doi.org/10.1038/s41390-018-0079-8.
Eriksen BH, Nestaas E, Hole T, Liestøl K, Støylen A, Fugelseth D. Longitudinal assessment of atrioventricular annulus excursion by grey-scale m-mode and colour tissue Doppler imaging in premature infants. Early Hum Dev. 2013;89(12):977–82. https://doi.org/10.1016/j.earlhumdev.2013.09.006.
Mandysová E, Mráz T, Táborský M, Niederle P. Reproducibility of tissue Doppler parameters of asynchrony in patients with advanced LV dysfunction. Eur J Echocardiogr. 2008;9(4):509–15. https://doi.org/10.1016/j.euje.2007.08.005.
Blessberger H, Binder T. Two dimensional speckle tracking echocardiography: basic principles. Heart 2010;96(9). https://doi.org/10.1136/hrt.2007.141002.
Geyer H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2010;23(4). https://doi.org/10.1016/j.echo.2010.02.015.
Leitman M, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2004;17(10). https://doi.org/10.1016/j.echo.2004.06.019.
Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2004;17(6). https://doi.org/10.1016/j.echo.2004.02.011.
Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R. Strain and strain rate imaging by echocardiography—basic concepts and clinical applicability. Curr Cardiol Rev. 2009;5(2). https://doi.org/10.2174/157340309788166642.
Hayat D, et al. Comparison of real-time three-dimensional speckle tracking to magnetic resonance imaging in patients with coronary heart disease. Am J Cardiol. 2012;109(2). https://doi.org/10.1016/j.amjcard.2011.08.030.
Suhling M, Arigovindan M, Jansen C, Hunziker P, Unser M. Myocardial motion analysis from B-mode echocardiograms. IEEE Trans Image Process. 2005;14(4). https://doi.org/10.1109/TIP.2004.838709.
Yu W, Yan P, Sinusas AJ, Thiele K, Duncan JS. Towards pointwise motion tracking in echocardiographic image sequences—comparing the reliability of different features for speckle tracking. Med Image Anal. 2006;10(4). https://doi.org/10.1016/j.media.2005.12.003.
Paragios N. A level set approach for shape-driven segmentation and tracking of the left ventricle. IEEE Trans Med Imaging 2003;22(6). https://doi.org/10.1109/TMI.2003.814785.
Elen A, et al. Three-dimensional cardiac strain estimation using spatio-temporal elastic registration of ultrasound images: a feasibility study. IEEE Trans Med Imaging 2008;27(11). https://doi.org/10.1109/TMI.2008.2004420.
Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452(7187):580–9. https://doi.org/10.1038/nature06917.
Epstein FH. MR in mouse models of cardiac disease. NMR Biomed. 2007;20(3):238–55. https://doi.org/10.1002/nbm.1152.
Savchenko A, et al. Graphene biointerfaces for optical stimulation of cells. Sci Adv. 4(5):eaat0351. https://doi.org/10.1126/sciadv.aat0351.
Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EHK. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305(5686):1007–9. https://doi.org/10.1126/science.1100035.
Weber M, Mickoleit M, Huisken J. Chapter 11—light sheet microscopy. Methods Cell Biol. 2014;123. Waters JC, Wittman T. Eds. Academic Press. 2014;193–215. https://doi.org/10.1016/B978-0-12-420138-5.00011-2.
Levoy M, Ng R, Adams A, Footer M, Horowitz M. Light field microscopy. ACM SIGGRAPH 2006 Papers, New York, NY, USA. 2006;924–934. https://doi.org/10.1145/1179352.1141976.
Prevedel R, et al. Simultaneous whole-animal 3D imaging of neuronal activity using light-field microscopy. Nat Methods 2014;11(7). https://doi.org/10.1038/nmeth.2964.
Pégard NC, Liu H-Y, Antipa N, Gerlock M, Adesnik H, Waller L. Compressive light-field microscopy for 3D neural activity recording. Optica. 2016;3(5):517–24. https://doi.org/10.1364/OPTICA.3.000517.
Cong L, et al. Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio). eLife 2017;6:e28158. https://doi.org/10.7554/eLife.28158.
Wagner N, et al. Instantaneous isotropic volumetric imaging of fast biological processes. Nat Methods 2019;16(6). https://doi.org/10.1038/s41592-019-0393-z.
• Wang Z, et al. Real-time volumetric reconstruction of biological dynamics with light-field microscopy and deep learning. Nat Methods 2021;18(5). https://doi.org/10.1038/s41592-021-01058-x. This study offers a compelling solution for investigating the dynamic properties and functions of the cardiovascular system based on light-field microscopy and an easily adoptable deep-learning framework.
Ding Y, et al. Integrating light-sheet imaging with virtual reality to recapitulate developmental cardiac mechanics. JCI Insight 2017;2(22). https://doi.org/10.1172/jci.insight.97180.
Wang Z, Ding Y, Satta S, Roustaei M, Fei P, Hsiai TK. A hybrid of light-field and light-sheet imaging to study myocardial function and intracardiac blood flow during zebrafish development. PLOS Comput Biol. 2021;17(7). https://doi.org/10.1371/journal.pcbi.1009175.
• Chen J, et al. Displacement analysis of myocardial mechanical deformation (DIAMOND) reveals segmental susceptibility to doxorubicin-induced injury and regeneration. JCI Insight 2019;4(8). https://doi.org/10.1172/jci.insight.125362. This study developed a semiautomated method to assess the displacement of regional myocardial deformation, providing a new biomechanical insight into the in vivo analysis of cardiac contractile function.
Brock KK, Sharpe MB, Dawson LA, Kim SM, Jaffray DA. Accuracy of finite element model-based multi-organ deformable image registration. Med Phys. 2005;32(6):1647–59. https://doi.org/10.1118/1.1915012.
Hill DL, Batchelor PG, Holden M, Hawkes DJ. Medical image registration. Phys Med Biol. 2001;46(3):R1-45. https://doi.org/10.1088/0031-9155/46/3/201.
Thirion JP. Image matching as a diffusion process: an analogy with Maxwell’s demons. Med Image Anal. 1998;2(3):243–60. https://doi.org/10.1016/s1361-8415(98)80022-4.
O’Shea C, et al. Cardiac optical mapping—state-of-the-art and future challenges. Int J Biochem Cell Biol. 2020;126: 105804. https://doi.org/10.1016/j.biocel.2020.105804.
Huang D, et al. Optical Coherence Tomography. Science. 1991;254(5035):1178–81. https://doi.org/10.1126/science.1957169.
Attizzani GF, Patrício L, Bezerra HG. Optical coherence tomography assessment of calcified plaque modification after rotational atherectomy. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2013;81(3). https://doi.org/10.1002/ccd.23385.
Wang S, Larina I. Live mechanistic assessment of localized cardiac pumping in mammalian tubular embryonic heart. J Biomed Opt. 2020;25(8). https://doi.org/10.1117/1.JBO.25.8.086001.
Lv J, et al. Hemispherical photoacoustic imaging of myocardial infarction: in vivo detection and monitoring. Eur Radiol. 2018;28(5). https://doi.org/10.1007/s00330-017-5209-x.
Hou J, et al. OCT Assessment of allograft vasculopathy in heart transplant recipients. JACC Cardiovasc Imaging. 2012;5(6):662–3. https://doi.org/10.1016/j.jcmg.2012.01.018.
Zhang C, Wang LV, Cheng Y-J, Chen J, Wickline SA. Label-free photoacoustic microscopy of myocardial sheet architecture. J Biomed Opt. 2012;17(6): 060506. https://doi.org/10.1117/1.JBO.17.6.060506.
Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335(6075):1458–62. https://doi.org/10.1126/science.1216210.
Fernandez-Gonzalez R, et al. PyJAMAS: open-source, multimodal segmentation and analysis of microscopy images. Bioinformatics. 2022;38(2):594–6. https://doi.org/10.1093/bioinformatics/btab589.
Wen C, et al. 3DeeCellTracker, a deep learning-based pipeline for segmenting and tracking cells in 3D time lapse images. eLife 2021;10:e59187. https://doi.org/10.7554/eLife.59187.
Beucher S. The watershed transformation applied to image segmentation. Scanning Microsc Suppl. 1992;299–314.
Wang MFZ, Hunter MV, Wang G, McFaul C, Yip CM, Fernandez-Gonzalez R. Automated cell tracking identifies mechanically oriented cell divisions during Drosophila axis elongation. Dev Camb Engl. 2017;144(7):1350–61. https://doi.org/10.1242/dev.141473.
Lindsey ML, Iyer RP, Jung M, DeLeon-Pennell KY, Ma Y. Matrix metalloproteinases as input and output signals for post-myocardial infarction remodeling. J Mol Cell Cardiol. 2016;91:134–40. https://doi.org/10.1016/j.yjmcc.2015.12.018.
Genet M, et al. Heterogeneous growth-induced prestrain in the heart. J Biomech. 2015;48(10):2080–9. https://doi.org/10.1016/j.jbiomech.2015.03.012.
Gupta A, Eisen HJ. Cardiac-oncology: management of the patient with heart failure after chemotherapy. In Heart Failure: A Comprehensive Guide to Pathophysiology and Clinical Care, H. Eisen, Ed. London: Springer. 2017,309–325. https://doi.org/10.1007/978-1-4471-4219-5_13.
Dobson R, et al. BSE and BCOS Guideline for Transthoracic Echocardiographic Assessment of Adult Cancer Patients Receiving Anthracyclines and/or Trastuzumab. JACC Cardio Oncol. 2021;3(1):1–16. https://doi.org/10.1016/j.jaccao.2021.01.011.
Čelutkienė J, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail. 2020;22(9):1504–24. https://doi.org/10.1002/ejhf.1957.
Zamorano JL, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–801. https://doi.org/10.1093/eurheartj/ehw211.
Plana JC, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2014;27(9):911–39. https://doi.org/10.1016/j.echo.2014.07.012.
Manrique CR, Park M, Tiwari N, Plana JC, Garcia MJ. Diagnostic strategies for early recognition of cancer therapeutics–related cardiac dysfunction. Clin Med Insights Cardiol. 2017;11. https://doi.org/10.1177/1179546817697983.
Perez IE, Taveras Alam S, Hernandez GA, Sancassani R. Cancer Therapy-related cardiac dysfunction: an overview for the clinician. Clin Med Insights Cardiol. 2019;13. https://doi.org/10.1177/1179546819866445.
Campbell JM, Hartjes KA, Nelson TJ, Xu X, Ekker SC. New and TALENted genome engineering toolbox. Circ Res. 2013;113(5):571–87. https://doi.org/10.1161/CIRCRESAHA.113.301765.
Kim J-D, Lee H-W, Jin S-W. Diversity is in my veins. Arterioscler Thromb Vasc Biol. 2014;34(9):1838–45. https://doi.org/10.1161/ATVBAHA.114.303219.
Milan DJ, Jones IL, Ellinor PT, MacRae CA. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am J Physiol Heart Circ Physiol. 2006;291(1):H269-273. https://doi.org/10.1152/ajpheart.00960.2005.
Santoro MM. Antiangiogenic cancer drug using the zebrafish model. Arterioscler Thromb Vasc Biol. 2014;34(9):1846–53. https://doi.org/10.1161/ATVBAHA.114.303221.
Sedmera D, et al. Functional and morphological evidence for a ventricular conduction system in zebrafish and Xenopus hearts. Am J Physiol Heart Circ Physiol. 2003;284(4):H1152-1160. https://doi.org/10.1152/ajpheart.00870.2002.
Packard RRS, et al. Automated segmentation of light-sheet fluorescent imaging to characterize experimental doxorubicin-induced cardiac injury and repair. Sci Rep. 2017;7(1). https://doi.org/10.1038/s41598-017-09152-x.
Lamounier E, Bucioli A, Cardoso A, Andrade A, Soares A. On the use of Augmented Reality techniques in learning and interpretation of cardiologic data. Ann Int Conf IEEE Eng Med Biol. 2010;2010:610–3. https://doi.org/10.1109/IEMBS.2010.5628019.
Koger CR, Hassan SS, Yuan J, Ding Y. Virtual reality for interactive medical analysis. Front Virtual Real. 2022;3. Available: https://www.frontiersin.org/article/10.3389/frvir.2022.782854. Accessed 15 Mar 2022.
Abiri A, et al. Simulating developmental cardiac morphology in virtual reality using a deformable image registration approach. Ann Biomed Eng. 2018;46(12):2177–88. https://doi.org/10.1007/s10439-018-02113-z.
Kaluzynski K, Chen X, Emelianov SY, Skovoroda AR, O’Donnell M. Strain rate imaging using two-dimensional speckle tracking. IEEE Trans Ultrason Ferroelectr Freq Control. 2001;48(4):1111–23. https://doi.org/10.1109/58.935730.
Helle-Valle T, et al. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112(20):3149–56. https://doi.org/10.1161/CIRCULATIONAHA.104.531558.
Acknowledgements
This work was supported by NIH R00 HL148493 (YD) and the University of Texas at Dallas.
Author information
Authors and Affiliations
Contributions
The authors would like to express gratitude to all other lab members for constructive discussion. All the authors contributed to the content in this review.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Heart Failure
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Alexander, R.V., Yuan, J. et al. Computational Analysis of Cardiac Contractile Function. Curr Cardiol Rep 24, 1983–1994 (2022). https://doi.org/10.1007/s11886-022-01814-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01814-1