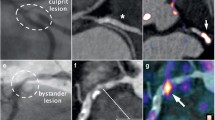
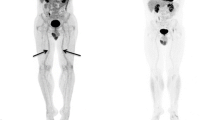
Abstract
Purpose of Review
In this review, we focus on the clinical and epidemiological studies pertaining to systemic and vascular inflammation by positron emission tomography (PET) in patients with chronic inflammatory conditions such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), human immunodeficiency virus (HIV), and psoriasis to highlight the importance of chronic systemic inflammation on vascular inflammation by PET in these disease states.
Recent Findings
Recent clinical and translation advancements have demonstrated the durable relationship between chronic systemic inflammation and cardiovascular disease (CVD). In chronic inflammatory states, this relationship is robustly evident in the form of increased vascular inflammation, yet traditional risk estimates often underestimate the subclinical cardiovascular risk conferred by chronic inflammation. PET has emerged as a novel, non-invasive imaging modality capable of both quantifying the degree of systemic and vascular inflammation and detecting residual inflammation prior to cardiovascular events.
Summary
We begin by demonstrating the role of inflammation in the pathogenesis of atherosclerosis, discussing how PET has been utilized to measure systemic and vascular inflammation and their effect on subclinical atherosclerosis, and finally reviewing recent applications of PET in constructing improved risk stratification for patients at high risk for stroke and CVD.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
World Health Organization - Cardiovascular Diseases Fact Sheet [Internet]. 2021. Available from: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds.
Patel NH, Dey AK, Sorokin AV, Teklu M, Petrole R, Zhou W, et al. Chronic inflammatory diseases and coronary heart disease: insights from cardiovascular CT. J Cardiovasc Comput Tomogr [Internet]. 2021. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1934592521000861.
Torres T, Sales R, Vasconcelos C, Martins da Silva B, Selores M. Framingham Risk Score underestimates cardiovascular disease risk in severe psoriatic patients: implications in cardiovascular risk factors management and primary prevention of cardiovascular disease. J Dermatol [Internet]. 2013;40:923–6. Available from: https://onlinelibrary.wiley.com/doi/10.1111/1346-8138.12267.
Mehta NN, Yu Y, Pinnelas R, Krishnamoorthy P, Shin DB, Troxel AB, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med [Internet]. 2011;124:775.e1–775.e6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002934311003275.
Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J [Internet]. 2018;39:3499–507. Available from: https://academic.oup.com/eurheartj/article/39/38/3499/5078464.
Reddy AS, Uceda DE, Al Najafi M, Dey AK, Mehta NN. PET scan with fludeoxyglucose/computed tomography in low-grade vascular inflammation. PET Clin [Internet]. 2020;15:207–13. Available from: https://linkinghub.elsevier.com/retrieve/pii/S155685981930104X.
Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers. Circ Cardiovasc Imaging [Internet]. 2018;11. Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.117.007394.
Fernández-Friera L, Fuster V, López-Melgar B, Oliva B, Sánchez-González J, Macías A, et al. Vascular inflammation in subclinical atherosclerosis detected by hybrid PET/MRI. J Am Coll Cardiol [Internet]. 2019;73:1371–82. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109719304127.
Teague HL, Ahlman MA, Alavi A, Wagner DD, Lichtman AH, Nahrendorf M, et al. Unraveling vascular inflammation. J Am Coll Cardiol [Internet]. 2017;70:1403–12. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109717389647.
Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J [Internet]. 2015;36:482–9. Available from: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehu403.
Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res [Internet]. 2019;124:315–27. Available from: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.118.313591.
Aizaz M, Moonen RPM, van der Pol JAJ, Prieto C, Botnar RM, Kooi ME. PET/MRI of atherosclerosis. Cardiovasc Diagn Ther [Internet]. 2020;10:1120–39. Available from: http://cdt.amegroups.com/article/view/42236/html.
Lee YK, Kwak HS, Chung GH, Hwang SB. Lipid-rich necrotic core of basilar artery atherosclerotic plaque: contrast-enhanced black blood imaging on vessel wall imaging. Diagnostics [Internet]. 2019;9:69. Available from: https://www.mdpi.com/2075-4418/9/3/69.
Joshi N V, Vesey AT, Williams MC, Shah AS V, Calvert PA, Craighead FHM, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet [Internet]. 2014;383:705–13. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673613617547.
Høilund-Carlsen PF, Sturek M, Alavi A, Gerke O. Atherosclerosis imaging with 18F-sodium fluoride PET: state-of-the-art review. Eur J Nucl Med Mol Imaging [Internet]. 2020;47:1538–51. Available from: http://link.springer.com/10.1007/s00259-019-04603-1.
Mayer M, Borja AJ, Hancin EC, Auslander T, Revheim M-E, Moghbel MC, et al. Imaging atherosclerosis by PET, with emphasis on the role of FDG and NaF as potential biomarkers for this disorder. Front Physiol [Internet]. 2020;11. Available from: https://www.frontiersin.org/articles/10.3389/fphys.2020.511391/full.
Abdelbaky A, Tawakol A. Noninvasive positron emission tomography imaging of coronary arterial inflammation. Curr Cardiovasc Imaging Rep [Internet]. 2011;4:41–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21379370.
Alie N, Eldib M, Fayad ZA, Mani V. Inflammation, atherosclerosis, and coronary artery disease: PET/CT for the evaluation of atherosclerosis and inflammation. Clin Med Insights Cardiol [Internet]. 2014;8s3:CMC.S17063. Available from: http://journals.sagepub.com/doi/10.4137/CMC.S17063.
Lairez O, Hyafil F. A clinical role of PET in atherosclerosis and vulnerable plaques? Semin Nucl Med [Internet]. 2020;50:311–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0001299820300295.
McKenney-Drake ML, Moghbel MC, Paydary K, Alloosh M, Houshmand S, Moe S, et al. 18F-NaF and 18F-FDG as molecular probes in the evaluation of atherosclerosis. Eur J Nucl Med Mol Imaging [Internet]. 2018;45:2190–200. Available from: http://link.springer.com/10.1007/s00259-018-4078-0.
Zhou W, Dey A, Manyak G, Teklu M, Patel N, Teague H, et al. The application of molecular imaging to advance translational research in chronic inflammation. J Nucl Cardiol [Internet]. 2020; Available from: http://link.springer.com/10.1007/s12350-020-02439-z.
Mehta NN, Torigian DA, Gelfand JM, Saboury B, Alavi A. Quantification of atherosclerotic plaque activity and vascular inflammation using [18-F] Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (FDG-PET/CT). J Vis Exp [Internet]. 2012; Available from: http://www.jove.com/video/3777/.
Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol [Internet]. 2015;35:2667–76. Available from: https://www.ahajournals.org/doi/10.1161/ATVBAHA.115.306460.
Pahk K, Kim EJ, Joung C, Seo HS, Kim S. Association of glucose uptake of visceral fat and acute myocardial infarction: a pilot 18F-FDG PET/CT study. Cardiovasc Diabetol [Internet]. 2020;19:145. Available from: https://cardiab.biomedcentral.com/articles/10.1186/s12933-020-01115-3.
Gelfand JM, Shin DB, Duffin KC, Armstrong AW, Blauvelt A, Tyring SK, et al. A randomized placebo-controlled trial of secukinumab on aortic vascular inflammation in moderate-to-severe plaque psoriasis (VIP-S). J Invest Dermatol [Internet]. 2020;140:1784–93.e2. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022202X20301573.
Gelfand JM, Shin DB, Alavi A, Torigian DA, Werner T, Papadopoulos M, et al. A phase IV, Randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U trial). J Invest Dermatol [Internet]. 2020;140:85–93.e2. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022202X19325370.
Gonzalez-Cantero A, Teklu M, Sorokin A V., Prussick R, González-Cantero J, Martin- Rodriguez JL, et al. Subclinical liver disease is associated with subclinical atherosclerosis in psoriasis: results from two observational studies. J Invest Dermatol [Internet]. 2021. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022202X21014615.
Sathekge M, Maes A, Kgomo M, Stolz A, Ankrah A, Van de Wiele C. Evaluation of glucose uptake by skeletal muscle tissue and subcutaneous fat in HIV-infected patients with and without lipodystrophy using FDG-PET. Nucl Med Commun [Internet]. 2010;31:311–4. Available from: https://journals.lww.com/00006231-201004000-00008.
Hammoud DA, Boulougoura A, Papadakis GZ, Wang J, Dodd LE, Rupert A, et al. Increased metabolic activity on 18F-fluorodeoxyglucose positron emission tomography–computed tomography in human immunodeficiency virus–associated immune reconstitution inflammatory syndrome. Clin Infect Dis [Internet]. 2019;68:229–38. Available from: https://academic.oup.com/cid/article/68/2/229/5095310.
Goyal A, Dey AK, Chaturvedi A, Elnabawi YA, Aberra TM, Chung JH, et al. Chronic stress-related neural activity associates with subclinical cardiovascular disease in psoriasis. JACC Cardiovasc Imaging [Internet]. 2020;13:465–77. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1936878X18309203.
Wang H, Zhu W, Ren S, Kong Y, Huang Q, Zhao J, et al. 68Ga-FAPI-04 Versus 18F-FDG PET/CT in the detection of hepatocellular carcinoma. Front Oncol [Internet]. 2021;11:693640. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34249748.
Toutouzas K, Skoumas J, Koutagiar I, Benetos G, Pianou N, Georgakopoulos A, et al. Vascular inflammation and metabolic activity in hematopoietic organs and liver in familial combined hyperlipidemia and heterozygous familial hypercholesterolemia. J Clin Lipidol [Internet]. 12:33–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29174439.
Hellwig S, Frings L, Bormann T, Vach W, Buchert R, Meyer PT. Amyloid imaging for differential diagnosis of dementia: incremental value compared to clinical diagnosis and [18F]FDG PET. Eur J Nucl Med Mol Imaging [Internet]. 2019;46:312–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30094462.
Rose S, Sheth NH, Baker JF, Ogdie A, Raper A, Saboury B, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis [Internet]. 2013;3:273–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24224139.
Carlucci PM, Purmalek MM, Dey AK, Temesgen-Oyelakin Y, Sakhardande S, Joshi AA, et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight [Internet]. 2018;3. Available from: https://insight.jci.org/articles/view/99276.
• Taglieri N, Bonfiglioli R, Bon I, Malosso P, Corovic A, Bruno M, et al. Pattern of arterial inflammation and inflammatory markers in people living with HIV compared with uninfected people. J Nucl Cardiol [Internet]. 2021. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33569752. One of the first studies to report the heightened FDG uptake in the thoracic aorta predicted independently by HIV infection in subjects with cardiovascular risk.
Groenendyk JW, Shukla P, Dey AK, Elnabawi YA, Aksentijevich M, Choi H, et al. Association of aortic vascular uptake of 18FDG by PET/CT and aortic wall thickness by MRI in psoriasis: a prospective observational study. Eur J Nucl Med Mol Imaging [Internet]. 2019;46:2488–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31385013.
Joshi AA, Lerman JB, Dey AK, Sajja AP, Belur AD, Elnabawi YA, et al. Association between aortic vascular inflammation and coronary artery plaque characteristics in psoriasis. JAMA Cardiol [Internet]. 2018;3:949. Available from: http://cardiology.jamanetwork.com/article.aspx?doi=10.1001/jamacardio.2018.2769.
Nowak M, Carrasquillo JA, Yarboro CH, Bacharach SL, Whatley M, Valencia X, et al. A pilot study of the use of 2-[18F]-fluoro-2-deoxy-D-glucose-positron emission tomography to assess the distribution of activated lymphocytes in patients with systemic lupus erythematosus. Arthritis Rheum [Internet]. 2004;50:1233–8. Available from: https://onlinelibrary.wiley.com/doi/10.1002/art.20150.
Makis W, Ciarallo A, Gonzalez-Verdecia M, Probst S. Systemic lupus erythematosus associated pitfalls on 18F-FDG PET/CT: reactive follicular hyperplasia, Kikuchi-Fujimoto disease, inflammation and lymphoid hyperplasia of the spleen mimicking lymphoma. Nucl Med Mol Imaging (2010) [Internet]. 2018;52:74–9. Available from: http://link.springer.com/10.1007/s13139-017-0471-z.
Girard A, Ohnona J, Bernaudin J-F, Montravers F, Bachmeyer C. Generalized lymph node FDG uptake as the first manifestation of systemic lupus erythematosus. Clin Nucl Med [Internet]. 2017;42:787–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28806242.
Kaiser H, Kvist-Hansen A, Krakauer M, Gørtz PM, Henningsen KMA, Wang X, et al. Association between vascular inflammation and inflammation in adipose tissue, spleen, and bone marrow in patients with psoriasis. Life [Internet]. 2021;11:305. Available from: https://www.mdpi.com/2075-1729/11/4/305.
Lawal IO, Ankrah AO, Popoola GO, Lengana T, Sathekge MM. Arterial inflammation in young patients with human immunodeficiency virus infection: a cross-sectional study using F-18 FDG PET/CT. J Nucl Cardiol [Internet]. 2019;26:1258–65. Available from: http://link.springer.com/10.1007/s12350-018-1207-x.
Egeberg A, Skov L, Joshi AA, Mallbris L, Gislason GH, Wu JJ, et al. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events. J Am Acad Dermatol [Internet]. 2017;77:650–656.e3. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0190962217319254.
Longenecker CT, Sullivan CE, Morrison J, Hileman CO, Zidar DA, Gilkeson R, et al. The effects of HIV and smoking on aortic and splenic inflammation. AIDS [Internet]. 2018;32:89–94. Available from: https://journals.lww.com/00002030-201801020-00010.
Tawakol A, Lo J, Zanni M V., Marmarelis E, Ihenachor EJ, MacNabb M, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. JAIDS J Acquir Immune Defic Syndr [Internet]. 2014;66:164–71. Available from: https://journals.lww.com/00126334-201406010-00008.
Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R. Pathophysiology of atherosclerosis plaque progression. Hear Lung Circ [Internet]. 2013;22:399–411. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1443950613000711.
Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation [Internet]. 2020;141:1452–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32174130.
Karpouzas GA, Ormseth SR, Hernandez E, Budoff MJ. Biologics may prevent cardiovascular events in rheumatoid arthritis by inhibiting coronary plaque formation and stabilizing high-risk lesions. Arthritis Rheumatol (Hoboken, NJ) [Internet]. 2020;72:1467–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32319221.
Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, et al. Association between skin and aortic vascular inflammation in patients with psoriasis. JAMA Cardiol [Internet]. 2017;2:1013. Available from: http://cardiology.jamanetwork.com/article.aspx?doi=10.1001/jamacardio.2017.1213.
Amigues I, Tugcu A, Russo C, Giles JT, Morgenstein R, Zartoshti A, et al. Myocardial inflammation, measured using 18‐fluorodeoxyglucose positron emission tomography with computed tomography, is associated with disease activity in rheumatoid arthritis. Arthritis Rheumatol [Internet]. 2019;71:496–506. Available from: https://onlinelibrary.wiley.com/doi/10.1002/art.40771.
Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation. J Am Coll Cardiol [Internet]. 2013;62:909–17. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109713020822.
•• Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res [Internet]. 2019;115:721–8. Available from: https://academic.oup.com/cardiovascres/article/115/4/721/5306384. Findings from this study illustrate that non-calcified coronary plaque may decrease in psoriasis with treatment by biologic therapy.
Khan A, Arbab-Zadeh A, Kiani AN, Magder LS, Petri M. Progression of noncalcified and calcified coronary plaque by CT angiography in SLE. Rheumatol Int [Internet]. 2017;37:59–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27882428.
Ahlman MA, Maass-Moreno R, Grayson PC. Reply LTE, On semi-quantitative methods for assessing vascular 18 FDG-PET activity in large-vessel vasculitis. J Nucl Med [Internet]. 2021;jnumed.121.263158. Available from: http://jnm.snmjournals.org/lookup/doi/10.2967/jnumed.121.263158.
Huet P, Burg S, Le Guludec D, Hyafil F, Buvat I. Variability and uncertainty of 18 F-FDG PET imaging protocols for assessing inflammation in atherosclerosis: suggestions for improvement. J Nucl Med [Internet]. 2015;56:552–9. Available from: http://jnm.snmjournals.org/lookup/doi/10.2967/jnumed.114.142596.
Chen W, Dilsizian V. PET Assessment of vascular inflammation and atherosclerotic plaques: SUV or TBR? J Nucl Med [Internet]. 2015;56:503–4. Available from: http://jnm.snmjournals.org/lookup/doi/10.2967/jnumed.115.154385.
Vancheri F, Longo G, Vancheri S, Henein M. Coronary microvascular dysfunction. J Clin Med [Internet]. 2020;9:2880. Available from: https://www.mdpi.com/2077-0383/9/9/2880.
Zhou W, Bajaj N, Gupta A, Sun Y-P, Divakaran S, Bibbo C, et al. Coronary microvascular dysfunction, left ventricular remodeling, and clinical outcomes in aortic stenosis. J Nucl Cardiol [Internet]. 2021;28:579–88. Available from: https://link.springer.com/10.1007/s12350-019-01706-y.
Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol [Internet]. 2018;72:2625–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30466521.
Wan N, Travin MI. Cardiac PET assessment of myocardial microvascular flow may help identify subclinical left ventricular dysfunction and increased risk from aortic stenosis. J Nucl Cardiol [Internet]. 2021;28:589–93. Available from: https://link.springer.com/10.1007/s12350-019-01759-z.
Weber B, Perez-Chada LM, Divakaran S, Brown JM, Taqueti V, Dorbala S, et al. Coronary microvascular dysfunction in patients with psoriasis. J Nucl Cardiol [Internet]. 2020. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32419071.
• Weber BN, Stevens E, Barrett L, Bay C, Sinnette C, Brown JM, et al. Coronary microvascular dysfunction in systemic lupus erythematosus. J Am Heart Assoc [Internet]. 2021;10:e018555. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34132099. One of the most recent assessments of coronary microvascular dysfunction in SLE, demonstrating that SLE is associated with an elevated presence of coronary dysfunction that is unaccounted for by coronary risk factors and atherosclerosis.
Campbell BC V., De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Prim [Internet]. 2019;5:70. Available from: http://www.nature.com/articles/s41572-019-0118-8.
Ravikanth R. Role of 18 F-FDG positron emission tomography in carotid atherosclerotic plaque imaging: a systematic review. World J Nucl Med [Internet]. 2020;19:327. Available from: http://www.wjnm.org/text.asp?2020/19/4/327/290360.
Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol [Internet]. 2006;48:1818–24. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109706019802.
Kelly PJ, Camps-Renom P, Giannotti N, Martí-Fàbregas J, Murphy S, McNulty J, et al. Carotid plaque inflammation imaged by 18 F-fluorodeoxyglucose positron emission tomography and risk of early recurrent stroke. Stroke [Internet]. 2019;50:1766–73. Available from: https://www.ahajournals.org/doi/10.1161/STROKEAHA.119.025422.
Mikail N, Meseguer E, Lavallée P, Klein I, Hobeanu C, Guidoux C, et al. Evaluation of non-stenotic carotid atherosclerotic plaques with combined FDG-PET imaging and CT angiography in patients with ischemic stroke of unknown origin. J Nucl Cardiol [Internet]. 2021. Available from: http://link.springer.com/10.1007/s12350-020-02511-8.
Teklu M, Mehta NN. FDG-PET in ischemic strokes of unknown origin: have we found the needle in the haystack? J Nucl Cardiol [Internet]. 2021. Available from: http://link.springer.com/10.1007/s12350-021-02598-7.
Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med [Internet]. 2008;358:1336–45. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa072100.
Shao J-S, Cheng S-L, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification. Hypertension [Internet]. 2010;55:579–92. Available from: https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.109.134205.
Hutcheson JD, Blaser MC, Aikawa E. Giving calcification its due: recognition of a diverse disease. Circ Res [Internet]. 2017;120:270–3. Available from: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.116.310060.
Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis. J Am Coll Cardiol [Internet]. 2015;66:561–77. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109715027266.
Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, et al. Focal arterial inflammation precedes subsequent calcification in the same location. Circ Cardiovasc Imaging [Internet]. 2013;6:747–54. Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.113.000382.
Blau M, Ganatra R, Bender MA. 18F-fluoride for bone imaging. Semin Nucl Med [Internet]. 1972;2:31–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0001299872800059.
Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JLE, Dweck MR, et al. Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography. Nat Commun [Internet]. 2015;6:7495. Available from: http://www.nature.com/articles/ncomms8495.
Reith S, Milzi A, Dettori R, Marx N, Burgmaier M. Predictors for target lesion microcalcifications in patients with stable coronary artery disease: an optical coherence tomography study. Clin Res Cardiol [Internet]. 2018;107:763–71. Available from: http://link.springer.com/10.1007/s00392-018-1243-1.
Nakamoto Y, Kitagawa T, Sasaki K, Tatsugami F, Awai K, Hirokawa Y, et al. Clinical implications of 18F-sodium fluoride uptake in subclinical aortic valve calcification: its relation to coronary atherosclerosis and its predictive value. J Nucl Cardiol [Internet]. 2021;28:1522–31. Available from: https://link.springer.com/10.1007/s12350-019-01879-6.
Bellinge JW, Francis RJ, Lee SC, Phillips M, Rajwani A, Lewis JR, et al. 18 F-Sodium fluoride positron emission tomography activity predicts the development of new coronary artery calcifications. Arterioscler Thromb Vasc Biol [Internet]. 2020. Available from: https://www.ahajournals.org/doi/10.1161/ATVBAHA.120.315364.
Dweck MR, Jenkins WSA, Vesey AT, Pringle MAH, Chin CWL, Malley TS, et al. 18F-Sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging [Internet]. 2014;7:371–8. Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.113.001508.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Mehta is a full-time US government employee and has received research grants from Abbvie, Celgene, AstraZeneca, Janssen, Novartis, and Abcentra, outside the submitted work. The other authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nuclear Cardiology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parel, P.M., Berg, A.R., Hong, C.G. et al. Updates in the Impact of Chronic Systemic Inflammation on Vascular Inflammation by Positron Emission Tomography (PET). Curr Cardiol Rep 24, 317–326 (2022). https://doi.org/10.1007/s11886-022-01651-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01651-2