Abstract
Purpose of Review
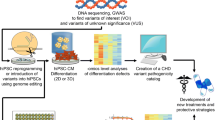
Heart development is a meticulously coordinated process that involves the specification of two distinct populations of cardiac progenitor cells, namely the first and the second heart field. Disruption of heart field progenitors can result in congenital heart defects. In this review, we aim to describe the signaling pathways and transcription factors that link heart field development and congenital heart disease.
Recent Findings
Single-cell transcriptomics, lineage-tracing mouse models, and stem cell-based in vitro modeling of cardiogenesis have significantly improved the spatiotemporal characterization of cardiac progenitors. Additionally, novel functional genomic studies have now linked more genetic variants with congenital heart disease.
Summary
Dysregulation of cardiac progenitor cells causes malformations that can be lethal. Ongoing research will continue to shed light on cardiac morphogenesis and help us better understand and treat patients with congenital heart disease.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Galdos FX, Guo Y, Paige SL, VanDusen NJ, Wu SM, Pu WT. Cardiac regeneration: lessons from development. Circ Res. 2017;120(6):941–59. https://doi.org/10.1161/CIRCRESAHA.116.309040This is a comprehensive review of cardiac development, heart regeneration and cardiomyocyte maturation.
•• Meilhac SM, Buckingham ME. The deployment of cell lineages that form the mammalian heart. Nat Rev Cardiol. 2018;15(11):705–24. https://doi.org/10.1038/s41569-018-0086-9This is an updated and thorough review on heart development
• Christoffels V, Jensen B. Cardiac morphogenesis: specification of the four-chambered heart. Cold Spring Harb Perspect Biol. 2020;12(10). https://doi.org/10.1101/cshperspect.a037143This is a recent review on cardiac morphogenesis with a focus on cardiac valve formation and the development of conduction system.
Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900. https://doi.org/10.1016/s0735-1097(02)01886-7.
Khoshnood B, Lelong N, Houyel L, Thieulin AC, Jouannic JM, Magnier S, et al. Prevalence, timing of diagnosis and mortality of newborns with congenital heart defects: a population-based study. Heart. 2012;98(22):1667–73. https://doi.org/10.1136/heartjnl-2012-302543.
Tennant PW, Pearce MS, Bythell M, Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet. 2010;375(9715):649–56. https://doi.org/10.1016/S0140-6736(09)61922-X.
Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9(17):2105–16. https://doi.org/10.1101/gad.9.17.2105.
Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1(1):37–49. https://doi.org/10.1016/s1534-5807(01)00017-x.
Cornell RA, Kimelman D. Activin-mediated mesoderm induction requires FGF. Development. 1994;120(2):453–62.
Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell. 2002;3(5):745–56. https://doi.org/10.1016/s1534-5807(02)00321-0.
Costello I, Pimeisl IM, Drager S, Bikoff EK, Robertson EJ, Arnold SJ. The T-box transcription factor Eomesodermin acts upstream of Mesp1 to specify cardiac mesoderm during mouse gastrulation. Nat Cell Biol. 2011;13(9):1084–91. https://doi.org/10.1038/ncb2304.
Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126(15):3437–47.
• Devine WP, Wythe JD, George M, Koshiba-Takeuchi K, Bruneau BG. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLife. 2014;3. https://doi.org/10.7554/eLife.03848This is a very important study reporting on the very early specification of cardiac progenitors.
• Lescroart F, Wang X, Lin X, Swedlund B, Gargouri S, Sanchez-Danes A, et al. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science. 2018;359(6380):1177–81. https://doi.org/10.1126/science.aao4174Single-cell RNA-Seq paper supporting very early specification of cardiac progenitors.
•• Kelly RG, Buckingham ME, Moorman AF. Heart fields and cardiac morphogenesis. Cold Spring Harb Perspect Med. 2014;4(10). https://doi.org/10.1101/cshperspect.a015750This is a comprehensive review specifically focusing on the development of two heart fields.
Spater D, Abramczuk MK, Buac K, Zangi L, Stachel MW, Clarke J, et al. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat Cell Biol. 2013;15(9):1098–106. https://doi.org/10.1038/ncb2824.
Ivanovitch K, Soro-Barrio P, Chakravarty P, Jones RA, Mousavy Gharavy SN, Stamataki D, et al. Ventricular, atrial and outflow tract heart progenitors arise from spatially and molecularly distinct regions of the primitive streak. bioRxiv. 2020:2020.07.12.198994. https://doi.org/10.1101/2020.07.12.198994.
Fujii M, Sakaguchi A, Kamata R, Nagao M, Kikuchi Y, Evans SM, et al. Sfrp5 identifies murine cardiac progenitors for all myocardial structures except for the right ventricle. Nat Commun. 2017;8:14664. https://doi.org/10.1038/ncomms14664.
Lescroart F, Chabab S, Lin X, Rulands S, Paulissen C, Rodolosse A, et al. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat Cell Biol. 2014;16(9):829–40. https://doi.org/10.1038/ncb3024.
Andersen P, Tampakakis E, Jimenez DV, Kannan S, Miyamoto M, Shin HK, et al. Precardiac organoids form two heart fields via Bmp/Wnt signaling. Nat Commun. 2018;9(1):3140. https://doi.org/10.1038/s41467-018-05604-8.
Watanabe Y, Zaffran S, Kuroiwa A, Higuchi H, Ogura T, Harvey RP, et al. Fibroblast growth factor 10 gene regulation in the second heart field by Tbx1, Nkx2-5, and Islet1 reveals a genetic switch for down-regulation in the myocardium. Proc Natl Acad Sci U S A. 2012;109(45):18273–80. https://doi.org/10.1073/pnas.1215360109.
Huynh T, Chen L, Terrell P, Baldini A. A fate map of Tbx1 expressing cells reveals heterogeneity in the second cardiac field. Genesis. 2007;45(7):470–5. https://doi.org/10.1002/dvg.20317.
• Jia G, Preussner J, Chen X, Guenther S, Yuan X, Yekelchyk M, et al. Single cell RNA-seq and ATAC-seq analysis of cardiac progenitor cell transition states and lineage settlement. Nat Commun. 2018;9(1):4877. https://doi.org/10.1038/s41467-018-07307-6Important study using single-cell ATAC-Seq to reconstruct developmental trajectories of mouse cardiac progenitors based on open chromatin states.
• Akerberg BN, Pu WT. Genetic and epigenetic control of heart development. Cold Spring Harb Perspect Biol. 2020;12(7). https://doi.org/10.1101/cshperspect.a036756This is a recent review on epigenetic regulation of heart development.
•• Pierpont ME, Brueckner M, Chung WK, Garg V, Lacro RV, McGuire AL, et al. Genetic basis for congenital heart disease: Revisited: a scientific statement from the American Heart Association. Circulation. 2018;138(21):e653–711. https://doi.org/10.1161/CIR.0000000000000606This is the most recent scientific statement by the American Heart Association regarding the genetic basis of congenital heart disease.
•• Kalisch-Smith JI, Ved N, Sparrow DB. Environmental Risk Factors for Congenital Heart Disease. Cold Spring Harb Perspect Biol. 2020;12(3). https://doi.org/10.1101/cshperspect.a037234This is an updated review regarding the role of environmental risk factors in congenital heart disease.
•• Nees SN, Chung WK. Genetic basis of human congenital heart disease. Cold Spring Harb Perspect Biol. 2020;12(9). https://doi.org/10.1101/cshperspect.a036749This is an updated and comprehensive review about the genetics of congenital heart disease.
Bruneau BG, Logan M, Davis N, Levi T, Tabin CJ, Seidman JG, et al. Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev Biol. 1999;211(1):100–8. https://doi.org/10.1006/dbio.1999.9298.
Mazen I, Amin H, Kamel A, El Ruby M, Bignon-Topalovic J, Bashamboo A, et al. Homozygous mutation of the FGFR1 gene associated with congenital heart disease and 46,XY disorder of sex development. Sex Dev. 2016;10(1):16–22. https://doi.org/10.1159/000444948.
Zhou S, Wang Q, Meng Z, Peng J, Zhou Y, Song W, et al. Mutations in fibroblast growth factor (FGF8) and FGF10 identified in patients with conotruncal defects. J Transl Med. 2020;18(1):283. https://doi.org/10.1186/s12967-020-02445-2.
Qian B, Mo R, Da M, Peng W, Hu Y, Mo X. Common variations in BMP4 confer genetic susceptibility to sporadic congenital heart disease in a Han Chinese population. Pediatr Cardiol. 2014;35(8):1442–7. https://doi.org/10.1007/s00246-014-0951-1.
Zhu MJ, Ma XY, Ding PC, Tang HF, Peng R, Lu L, et al. Novel mutations of AXIN2 identified in a Chinese Congenital Heart Disease Cohort. J Hum Genet. 2019;64(5):427–35. https://doi.org/10.1038/s10038-019-0572-x.
Giannakou A, Sicko RJ, Kay DM, Zhang W, Romitti PA, Caggana M, et al. Copy number variants in hypoplastic right heart syndrome. Am J Med Genet A. 2018;176(12):2760–7. https://doi.org/10.1002/ajmg.a.40527.
• MacGrogan D, Munch J, de la Pompa JL. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat Rev Cardiol. 2018;15(11):685–704. https://doi.org/10.1038/s41569-018-0100-2This is a compreheensive review of Notch and its role in heart development and regeneration.
• Wiegering A, Ruther U, Gerhardt C. The role of Hedgehog signalling in the formation of the ventricular septum. J Dev Biol. 2017;5(4). https://doi.org/10.3390/jdb5040017. This review summarizes well the association of Hedgehog signaling and the development of ventricular septal defect.
Lu CX, Gong HR, Liu XY, Wang J, Zhao CM, Huang RT, et al. A novel HAND2 loss-of-function mutation responsible for tetralogy of Fallot. Int J Mol Med. 2016;37(2):445–51. https://doi.org/10.3892/ijmm.2015.2436.
Sun YM, Wang J, Qiu XB, Yuan F, Li RG, Xu YJ, et al. A HAND2 loss-of-function mutation causes familial ventricular septal defect and pulmonary stenosis. G3 (Bethesda). 2016;6(4):987–92. https://doi.org/10.1534/g3.115.026518.
Stevens KN, Hakonarson H, Kim CE, Doevendans PA, Koeleman BP, Mital S, et al. Common variation in ISL1 confers genetic susceptibility for human congenital heart disease. PLoS One. 2010;5(5):e10855. https://doi.org/10.1371/journal.pone.0010855.
Ma L, Wang J, Li L, Qiao Q, Di RM, Li XM, et al. ISL1 loss-of-function mutation contributes to congenital heart defects. Heart Vessel. 2019;34(4):658–68. https://doi.org/10.1007/s00380-018-1289-z.
• Chung IM, Rajakumar G. Genetics of congenital heart defects: the NKX2-5 gene, a Key Player. Genes (Basel). 2016;7(2). https://doi.org/10.3390/genes7020006This is a metanalysis demonstrating the association of Nkx2.5 variants and congenital heart defects.
Zhang Y, Ai F, Zheng J, Peng B. Associations of GATA4 genetic mutations with the risk of congenital heart disease: a meta-analysis. Medicine (Baltimore). 2017;96(18):e6857. https://doi.org/10.1097/MD.0000000000006857.
Qiao XH, Wang F, Zhang XL, Huang RT, Xue S, Wang J, et al. MEF2C loss-of-function mutation contributes to congenital heart defects. Int J Med Sci. 2017;14(11):1143–53. https://doi.org/10.7150/ijms.21353.
Lu CX, Wang W, Wang Q, Liu XY, Yang YQ. A novel MEF2C loss-of-function mutation associated with congenital double-outlet right ventricle. Pediatr Cardiol. 2018;39(4):794–804. https://doi.org/10.1007/s00246-018-1822-y.
Marguerie A, Bajolle F, Zaffran S, Brown NA, Dickson C, Buckingham ME, et al. Congenital heart defects in Fgfr2-IIIb and Fgf10 mutant mice. Cardiovasc Res. 2006;71(1):50–60. https://doi.org/10.1016/j.cardiores.2006.03.021.
Watanabe Y, Miyagawa-Tomita S, Vincent SD, Kelly RG, Moon AM, Buckingham ME. Role of mesodermal FGF8 and FGF10 overlaps in the development of the arterial pole of the heart and pharyngeal arch arteries. Circ Res. 2010;106(3):495–503. https://doi.org/10.1161/CIRCRESAHA.109.201665.
Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5(6):877–89. https://doi.org/10.1016/s1534-5807(03)00363-0.
Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, et al. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117(7):1794–804. https://doi.org/10.1172/JCI31731.
Urness LD, Bleyl SB, Wright TJ, Moon AM, Mansour SL. Redundant and dosage sensitive requirements for Fgf3 and Fgf10 in cardiovascular development. Dev Biol. 2011;356(2):383–97. https://doi.org/10.1016/j.ydbio.2011.05.671.
Park EJ, Watanabe Y, Smyth G, Miyagawa-Tomita S, Meyers E, Klingensmith J, et al. An FGF autocrine loop initiated in second heart field mesoderm regulates morphogenesis at the arterial pole of the heart. Development. 2008;135(21):3599–610. https://doi.org/10.1242/dev.025437.
Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, et al. Fgf8 is required for anterior heart field development. Development. 2006;133(12):2435–45. https://doi.org/10.1242/dev.02408.
Jain R, Li D, Gupta M, Manderfield LJ, Ifkovits JL, Wang Q, et al. HEART DEVELOPMENT. Integration of Bmp and Wnt signaling by Hopx specifies commitment of cardiomyoblasts. Science. 2015;348(6242):aaa6071. https://doi.org/10.1126/science.aaa6071.
Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A. 2007;104(47):18531–6. https://doi.org/10.1073/pnas.0703113104.
Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, et al. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128(5):947–59. https://doi.org/10.1016/j.cell.2007.01.042.
Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, et al. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci U S A. 2007;104(22):9313–8. https://doi.org/10.1073/pnas.0700923104.
McCulley DJ, Kang JO, Martin JF, Black BL. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Developmental Dynamics. 2008;237(11):3200–9. https://doi.org/10.1002/dvdy.21743.
Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, et al. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103(52):19812–7. https://doi.org/10.1073/pnas.0605768103.
Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10(2):91–103. https://doi.org/10.1038/nrm2618.
Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15(3):316–27. https://doi.org/10.1101/gad.855501.
Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11(8):951–7. https://doi.org/10.1038/ncb1906.
• Cohen ED, Miller MF, Wang Z, Moon RT, Morrisey EE. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development. 2012;139(11):1931–40. https://doi.org/10.1242/dev.069377. Landmark study on the role of non-canonical Wnts in the development of second heart field.
Bisson JA, Mills B, Paul Helt JC, Zwaka TP, Cohen ED. Wnt5a and Wnt11 inhibit the canonical Wnt pathway and promote cardiac progenitor development via the Caspase-dependent degradation of AKT. Dev Biol. 2015;398(1):80–96. https://doi.org/10.1016/j.ydbio.2014.11.015.
Souilhol C, Cormier S, Tanigaki K, Babinet C, Cohen-Tannoudji M. RBP-Jkappa-dependent notch signaling is dispensable for mouse early embryonic development. Mol Cell Biol. 2006;26(13):4769–74. https://doi.org/10.1128/MCB.00319-06.
De Zoysa P, Liu J, Toubat O, Choi J, Moon A, Gill PS, et al. Delta-like ligand 4-mediated Notch signaling controls proliferation of second heart field progenitor cells by regulating Fgf8 expression. Development. 2020;147(17). https://doi.org/10.1242/dev.185249.
•• Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell. 2001;106(2):781–92. https://doi.org/10.1016/S0092-8674(01)00385-3This is a landmark study regarding the role of Hedgehoog signaling in regulating left-right asymmetry.
Liu J, Cheng H, Xiang M, Zhou L, Wu B, Moskowitz IP, et al. Gata4 regulates hedgehog signaling and Gata6 expression for outflow tract development. PLoS Genet. 2019;15(5):e1007711. https://doi.org/10.1371/journal.pgen.1007711.
Rowton M, Perez-Cervantes C, Rydeen A, Hur S, Jacobs-Li J, Deng N, et al. Hedgehog signaling activates a heterochronic gene regulatory network to control differentiation timing across lineages. bioRxiv. 2020:270751. https://doi.org/10.1101/270751.
Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27(3):286–91. https://doi.org/10.1038/85845.
Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, et al. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410(6824):97–101. https://doi.org/10.1038/35065105.
• Chen L, Fulcoli FG, Tang S, Baldini A. Tbx1 regulates proliferation and differentiation of multipotent heart progenitors. Circ Res. 2009;105(9):842–51. https://doi.org/10.1161/CIRCRESAHA.109.200295This is a review regarding the role of Tbx1 during cardiac development.
Vitelli F, Lania G, Huynh T, Baldini A. Partial rescue of the Tbx1 mutant heart phenotype by Fgf8: genetic evidence of impaired tissue response to Fgf8. J Mol Cell Cardiol. 2010;49(5):836–40. https://doi.org/10.1016/j.yjmcc.2010.08.023.
Caprio C, Baldini A. p53 Suppression partially rescues the mutant phenotype in mouse models of DiGeorge syndrome. Proc Natl Acad Sci U S A. 2014;111(37):13385–90. https://doi.org/10.1073/pnas.1401923111.
Racedo SE, Hasten E, Lin M, Devakanmalai GS, Guo T, Ozbudak EM, et al. Reduced dosage of beta-catenin provides significant rescue of cardiac outflow tract anomalies in a Tbx1 conditional null mouse model of 22q11.2 deletion syndrome. PLoS Genet. 2017;13(3):e1006687. https://doi.org/10.1371/journal.pgen.1006687.
Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16(2):154–60. https://doi.org/10.1038/ng0697-154.
• de Soysa TY, Ranade SS, Okawa S, Ravichandran S, Huang Y, Salunga HT, et al. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature. 2019;572(7767):120–4. https://doi.org/10.1038/s41586-019-1414-xThis is a recent study using single cell transcriptomics to analyze disrupted cardiac progenitors and their association with congenital heart disease.
Togi K, Yoshida Y, Matsumae H, Nakashima Y, Kita T, Tanaka M. Essential role of Hand2 in interventricular septum formation and trabeculation during cardiac development. Biochem Biophys Res Commun. 2006;343(1):144–51. https://doi.org/10.1016/j.bbrc.2006.02.122.
Tsuchihashi T, Maeda J, Shin CH, Ivey KN, Black BL, Olson EN, et al. Hand2 function in second heart field progenitors is essential for cardiogenesis. Dev Biol. 2011;351(1):62–9. https://doi.org/10.1016/j.ydbio.2010.12.023.
Vo NK, Dalton RP, Liu N, Olson EN, Goodman RH. Affinity purification of microRNA-133a with the cardiac transcription factor, Hand2. Proc Natl Acad Sci U S A. 2010;107(45):19231–6. https://doi.org/10.1073/pnas.1013162107.
Anderson KM, Anderson DM, McAnally JR, Shelton JM, Bassel-Duby R, Olson EN. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature. 2016;539(7629):433–6. https://doi.org/10.1038/nature20128.
Laugwitz KL, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: a single source for heart lineages? Development. 2008;135(2):193–205. https://doi.org/10.1242/dev.001883.
Xiong H, Luo Y, Yue Y, Zhang J, Ai S, Li X, et al. Single-cell transcriptomics reveals chemotaxis-mediated intraorgan crosstalk during cardiogenesis. Circ Res. 2019;125(4):398–410. https://doi.org/10.1161/CIRCRESAHA.119.315243.
Vincentz JW, Barnes RM, Firulli BA, Conway SJ, Firulli AB. Cooperative interaction of Nkx2.5 and Mef2c transcription factors during heart development. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237(12):3809–19. https://doi.org/10.1002/dvdy.21803.
Lien CL, Wu C, Mercer B, Webb R, Richardson JA, Olson EN. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development. 1999;126(1):75–84.
Liberatore CM, Searcy-Schrick RD, Vincent EB, Yutzey KE. Nkx-2.5 gene induction in mice is mediated by a Smad consensus regulatory region. Dev Biol. 2002;244(2):243–56. https://doi.org/10.1006/dbio.2002.0604.
Barth JL, Clark CD, Fresco VM, Knoll EP, Lee B, Argraves WS, et al. Jarid2 is among a set of genes differentially regulated by Nkx2.5 during outflow tract morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239(7):2024–33. https://doi.org/10.1002/dvdy.22341.
Rana MS, Theveniau-Ruissy M, De Bono C, Mesbah K, Francou A, Rammah M, et al. Tbx1 coordinates addition of posterior second heart field progenitor cells to the arterial and venous poles of the heart. Circ Res. 2014;115(9):790–9. https://doi.org/10.1161/CIRCRESAHA.115.305020.
•• McCulley DJ, Black BL. Transcription factor pathways and congenital heart disease. Curr Top Dev Biol. 2012;100:253–77. https://doi.org/10.1016/B978-0-12-387786-4.00008-7This is a review focusing on transcriptional regulation of heart development.
•• Kathiriya IS, Nora EP, Bruneau BG. Investigating the transcriptional control of cardiovascular development. Circ Res. 2015;116(4):700–14. https://doi.org/10.1161/CIRCRESAHA.116.302832This is a thorough review regarding transcriptional regulatory networks that control heart development.
Xie L, Hoffmann AD, Burnicka-Turek O, Friedland-Little JM, Zhang K, Moskowitz IP. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev Cell. 2012;23(2):280–91. https://doi.org/10.1016/j.devcel.2012.06.006.
Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106(6):709–21. https://doi.org/10.1016/s0092-8674(01)00493-7.
Steimle JD, Moskowitz IP. TBX5: a key regulator of heart development. Curr Top Dev Biol. 2017;122:195–221. https://doi.org/10.1016/bs.ctdb.2016.08.008.
Lickert H, Takeuchi JK, Von Both I, Walls JR, McAuliffe F, Adamson SL, et al. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature. 2004;432(7013):107–12. https://doi.org/10.1038/nature03071.
Kappen C, Salbaum JM. Identification of regulatory elements in the Isl1 gene locus. Int J Dev Biol. 2009;53(7):935–46. https://doi.org/10.1387/ijdb.082819ck.
Zhou L, Liu J, Xiang M, Olson P, Guzzetta A, Zhang K, et al. Gata4 potentiates second heart field proliferation and Hedgehog signaling for cardiac septation. Proc Natl Acad Sci U S A. 2017;114(8):E1422–E31. https://doi.org/10.1073/pnas.1605137114.
Dai YS, Cserjesi P, Markham BE, Molkentin JD. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J Biol Chem. 2002;277(27):24390–8. https://doi.org/10.1074/jbc.M202490200.
Desjardins CA, Naya FJ. The function of the MEF2 family of transcription factors in cardiac development, cardiogenomics, and direct reprogramming. J Cardiovasc Dev Dis. 2016;3(3). https://doi.org/10.3390/jcdd3030026.
Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131(16):3931–42. https://doi.org/10.1242/dev.01256.
Pane LS, Zhang Z, Ferrentino R, Huynh T, Cutillo L, Baldini A. Tbx1 is a negative modulator of Mef2c. Hum Mol Genet. 2012;21(11):2485–96. https://doi.org/10.1093/hmg/dds063.
Klaus A, Muller M, Schulz H, Saga Y, Martin JF, Birchmeier W. Wnt/beta-catenin and Bmp signals control distinct sets of transcription factors in cardiac progenitor cells. Proc Natl Acad Sci U S A. 2012;109(27):10921–6. https://doi.org/10.1073/pnas.1121236109.
Vong LH, Ragusa MJ, Schwarz JJ. Generation of conditional Mef2cloxP/loxP mice for temporal- and tissue-specific analyses. Genesis. 2005;43(1):43–8. https://doi.org/10.1002/gene.20152.
Barnes RM, Harris IS, Jaehnig EJ, Sauls K, Sinha T, Rojas A, et al. MEF2C regulates outflow tract alignment and transcriptional control of Tdgf1. Development. 2016;143(5):774–9. https://doi.org/10.1242/dev.126383.
Acknowledgments
Given the space limitations, a more comprehensive review of heart fields was not possible. We also apologize to any authors of studies that we might have omitted. Dr. Emmanouil Tampakakis is supported by NHLBI HL-145135, AHA CDA34660077, and the Magic that Matters Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Regenerative Medicine
Rights and permissions
About this article
Cite this article
Miyamoto, M., Gangrade, H. & Tampakakis, E. Understanding Heart Field Progenitor Cells for Modeling Congenital Heart Diseases. Curr Cardiol Rep 23, 38 (2021). https://doi.org/10.1007/s11886-021-01468-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01468-5