Abstract
Purpose of Review
This review focuses on the complex relationship between inflammation and the onset of acute coronary syndrome and heart failure.
Recent Findings
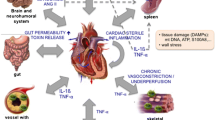
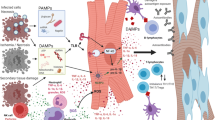
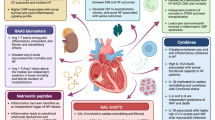
In the last few years, two important lines of research brought new and essential information to light in the pathogenesis of acute coronary syndrome: a) the understanding of the immune mediate mechanisms of inflammation in Ischemic Heart Disease (IHD) and b) evidence that the inflammatory mechanisms associated with atherosclerosis and its complications can be modulated by anti-inflammatory molecules. A large amount of data also suggests that inflammation is a major component in the development and exacerbation of heart failure (HF), in a symbiotic relationship. In particular, recent evidence underlies peculiar aspects of the phenomenon: oxidative stress and autophagy; DAMPS and TLR-4 signaling activation; different macrophages lineage and the contribution of NLRP-3 inflammasome; adaptive immune system. A possible explanation that could unify the pathogenic mechanism of these different conditions is the rising evidence that increased bowel permeability may allow translation of gut microbioma product into the circulation.
Summary
These findings clearly establish the role of inflammation as the great trigger for two of the major cardiovascular causes of death and morbidity. Further studies are needed, to better clarify the issue and to define more targeted approaches to reduce pathological inflammation while preserving the physiological one.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of Importance •• Of Major Importance
Rokitansky K. Handbuch der pathologoschen Anatomie. Braumiller & Seidel, Wien, pp. 1842-46.
Davies MJ. A macro and micro view of coronary vascular insult in ischemic heart disease. Circulation. 1990;82(3 Suppl):II38–46.
Serneri GG, Abbate R, Gori AM, Attanasio M, Martini F, Giusti B, et al. Transient intermittent lymphocyte activation is responsible for the instability of angina. Circulation. 1992;86(3):790–7.
Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med. 1994;331:417–24.
Biasucci LM, Vitelli A, Liuzzo G, Altamura S, Caligiuri G, Monaco C, et al. Elevated levels of interleukin-6 in unstable angina. Circulation. 1996;94(5):874–7.
Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, et al. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99(16):2079–84.
Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349(9050):462–6.
Ferreirós ER, Boissonnet CP, Pizarro R, Merletti PF, Corrado G, Cagide A, et al. Independent prognostic value of elevated C-reactive protein in unstable angina. Circulation. 1999;100(19):1958–63.
Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–9.
Crea F, Liuzzo G. Pathogenesis of acute coronary syndromes. J Am Coll Cardiol. 2013;61(1):1–11. doi:10.1016/j.jacc.2012.07.064.
Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34:719–28.
Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–13.
Giubilato S, Liuzzo G, Brugaletta S, et al. Expansion of CD4 + CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. Eur Heart J. 2011;32:1214–26.
Liuzzo G, Vallejo AN, Kopecky SL, et al. Molecular fingerprint of interferon-gamma signalling in unstable angina. Circulation. 2001;103:1509–14.
Liuzzo G, Biasucci LM, Trotta G, et al. Unusual CD4 + CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol. 2007;50:1450–8.
Liuzzo G, Trotta F, Pedicino D. Interleukin-17 in atherosclerosis and cardiovascular disease: the good, the bad, and the unknown. Eur Heart J. 2013;34:556–9.
Liu Z, Lu F, Pan H, Zhao Y, Wang S, Sun S, et al. Correlation of peripheral Th17 cells and Th17-associated cytokines to the severity of carotid artery plaque and its clinical implication. Atherosclerosis. 2012;221(1):232–41.
Basu R, Hatton RD, Weaver CT. The Th17 family: flexibility follows function. Immunol Rev. 2013;252(1):89–103.
Liuzzo G, Montone RA, Gabriele M, et al. Identification of unique adaptive immune system signature in acute coronary syndromes. Int J Cardiol. 2013;168:564–7.
Flego D, Severino A, Trotta F, et al. Increased PTPN22 expression and defective CREB activation impair regulatory T-cell differentiation in non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. 2015;65:1175–86.
Klingenberg R, Brokopp CE, Grivès A, et al. Clonal restriction and predominance of regulatory T cells in coronary thrombi of patients with acute coronary syndromes. Eur Heart J. 2015;36:1041–8.
Flego D, Severino A, Trotta F, et al. Altered CD31 expression and activity in helper T cells of acute coronary syndrome patients. Basic Res Cardiol. 2015;109:448.
Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–41.
Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ Res. 2011;108:985–95.
Ball RY, Stowers EC, Burton JH, Cary NR, Skepper JN, Mitchinson MJ. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54.
Libby et al. Inflammation and its resolution as determinants of acute coronary syndrome. Circ Res. 2014;114:1867–79.
Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1 moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118:145–56. This manuscript provides a valuable description of the CRP/IL-6/IL-1 axis in atherosclerotic development and novel therapeutic targets for atheroprotection.
Cannon CP, Braunwald E, et al. For the pravastatin or atorvastatin evaluation and infection therapy–thrombolysis in myocardial infarction 22 investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504.
Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49(21):2129–38. Epub 2007 Apr 30.
Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–68. doi:10.1161/CIRCRESAHA.116.302317. This review shows recent developments in the field of innate immunity and its role in the complex pathogenesis of heart failure.
Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–602. doi:10.1161/01.CIR.0000124490.27666.B2.
Selaas O, Nordal HH, Halse AK, Brun JG, Jonsson R, Brokstad KA. Serum markers in rheumatoid arthritis: a longitudinal study of patients undergoing infliximab treatment. Int J Rheumatol. 2015;2015:276815. doi:10.1155/2015/276815.
Liu L, Wang Y, Cao ZY, Wang MM, Liu XM, Gao T, et al. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J Cell Mol Med. 2015;19:2728–40. doi:10.1111/jcmm.12659.
Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–80. doi:10.1172/JCI6709.
Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res. 2004;95:700–7. doi:10.1161/01.RES.0000144175.70140.8c.
Gianni D, Li A, Tesco G, et al. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation. 2010;121:1216–26.
Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–24. doi:10.1038/nm1574.
Montaigne D, Marechal X, Coisne A, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130:554–64.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7.
Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–14.
La Rosa G, Biasucci LM. The gut microbiota and atherosclerosis: the state of the art and novel perspectives. Cardio Innov Appl. 2016;1(4):433–442(10). doi:10.15212/CVIA.2016.0027.
Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–82. doi:10.1161/CIRCRESAHA.113.301720.
Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon SI, et al. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H2238–47. doi:10.1152/ajpheart.00740.2007.
Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, et al. Dendritic cellinduced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9:1484–90. doi:10.1038/nm960.
Kobayashi K, Hernandez LD, Galan JE, Janeway Jr CA, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202.
Akita N, Tsujita M, Yokota T, Gonzalez FJ, Ohte N, Kimura G, et al. High density lipoprotein turnover is dependent on peroxisome proliferator-activated receptor alpha in mice. J Atheroscler Thromb. 2010;17:1149–59.
Genolet R, Wahli W, Michalik L. PPARs as drug targets to modulate inflammatory responses? Curr Drug Targets Inflamm Allergy. 2004;3:361–75.
Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi:10.1016/j.immuni.2013.11.019.
Takahashi M. NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J. 2014;55:101–5.
Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–61. doi:10.1146/annurev-cellbio-101011-155745.
Fedak PWM, Verma S, Weisel RD, Li R-K. Cardiac remodeling and failure. From molecules to Man (part 1). Cardio Pathol. 2005;14:1–11.
Fink SL, Cookson BT. Apoptosis, Pyroptosis, and Necrosis: Mechanistic Description of Dead and Dying Eukaryotic Cells. Infect Immun. 2005;73(4):1907–16. doi:10.1128/IAI.73.4.1907-1916.2005. PMC 1087413Freely accessible.
Bayeva M, Sawicki KT, Ardehali H. Taking diabetes to heart–deregulation of myocardial lipid metabolism in diabetic cardiomyopathy. J Am Heart Assoc. 2013;2.
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi:10.1161/CIRCHEARTFAILURE.109.931451.
Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserinepresenting liposomes improves infarct repair. Proc Natl Acad Sci U S A. 2011;108:1827–32. doi:10.1073/pnas.1015623108.
Bracey NA, Gershkovich B, Chun J, et al. Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome. J Biol Chem. 2014;289(28):19571–84. doi:10.1074/jbc.M114.550624.
Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–24. doi:10.1161/CIRCULATIONAHA.113.007101.
Liao YH, Xia N, Zhou SF, Tang TT, Yan XX, Lv BJ, et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. 2012;59:420–9. doi:10.1016/j.jacc.2011.10.863.
Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–94. doi:10.1016/j.immuni.2005.01.011.
Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, et al. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. 2010;106:1646–55. doi:10.1161/CIRCRESAHA.109.213157.
Wan E, Yeap XY, Dehn S, et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113:1004–12. doi:10.1161/CIRCRESAHA.113.301198.
Chung ES. Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40. doi:10.1161/01.CIR.0000077913.60364.D2.
Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi:10.1084/jem.20070885.
Torre-Amione G, Anker SD, Bourge RC, et al. Advanced Chronic Heart Failure CLinical Assessment of Immune Modulation Therapy Investigators. Results of a non-specific immunomodulation therapy in chronic heart failure (ACCLAIM trial): a placebo-controlled randomized trial. Lancet. 2008;371:228–36. doi:10.1016/S0140-6736(08)60134-8.
Poglajen G, Vrtovec B. Stem cell therapy for chronic heart failure. Curr Opin Cardiol. 2015;30:301–10. doi:10.1097/HCO.0000000000000167.
Tang XL, Li Q, Rokosh G, Sanganalmath SK, Chen N, Ou Q, et al. Long-term outcome of administration of c-kitPOS cardiac progenitor cells after acute myocardial infarction: transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ Res. 2016;118:1091–105. doi:10.1161/CIRCRESAHA.115.307647.
Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, et al. for the ixCELL-DCM Investigators. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet. 2016. doi:10.1016/S0140-6736(16)30137-4.
Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014;130(10):837–44. doi:10.1161/CIRCULATIONAHA.114.009990.
Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–20.
Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–75.
Cao F, Castrillo A, Tontonoz P, Re F, Byrne GI. Chlamydia pneumoniae– induced macrophage foam cell formation is mediated by Toll-like receptor 2. Infect Immun. 2007;75:753–9.
Ariza M, Williams M, Wong H. Targeting IL-17 in psoriasis: From cutaneous immunobiology to clinical application. Clin Immun. 2013;146:131–9. doi:10.1016/j.clim.2012.12.004.
Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: Rationale and Design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162:597–605. doi:10.1016/j.ahj.2011.06.012.
Abbate A, Kontos M, Grizzard J, Biondi-Zoccai GG, Van Tassell BW, Robati R, et al. Interleukin-1 Blockade With Anakinra to Prevent Adverse Cardiac Remodeling After Acute Myocardial Infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot Study). Am J Cardiol. 2010;105(10):1371–1377.e1. doi:10.1016/j.amjcard.2009.12.059.
McCartney SA, Vermi W, Lonardi S, Rossini C, Otero K, Calderon B, et al. RNA sensor-induced type I IFN prevents diabetes caused by a β cell-tropic virus in mice. J Clin Invest. 2011;121:1497–507. doi:10.1172/JCI44005.
Leblond AL, Klinkert K, Martin K, Turner EC, Kumar AH. BrowneT, Caplice NM. Systemic and Cardiac Depletion of M2 Macrophagethrough CSF-1R Signaling Inhibition Alters Cardiac Function PostMyocardial Infarction. PLoS One. 2015;10, e0137515. doi:10.1371/journal.pone.0137515.
Du CK, Morimoto S, Nishii K, et al. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185–94. doi:10.1161/CIRCRESAHA.106.146670.
Wen Y, Xu Y, Wang Y, Pinto JR, Potter JD, Kerrick WG. Functional effects of a restrictive-cardiomyopathy-linked cardiac troponin I mutation (R145W) in transgenic mice. J Mol Biol. 2009;392:1158–67. doi:10.1016/j.jmb.2009.07.080.
Morimoto S, Lu QW, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M, et al. Ca(2+)-desensitizing effect of a deletion mutation Delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:913–8. doi:10.1073/pnas.022628899.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Luigi M. Biasucci is a recipient of a research grant from Astra-Zeneca and fees for speeches from Siemens, Bayer, and Novartis.
Giulio La Rosa, Daniela Pedicino, Alessia D’Aiello, Mattia Galli, and Giovanna Liuzzo declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Luigi M. Biasucci and Giulio La Rosa equally contributed to the manuscript
This article is part of the Topical Collection on Management of Acute Coronary Syndromes
Rights and permissions
About this article
Cite this article
Biasucci, L.M., La Rosa, G., Pedicino, D. et al. Where Does Inflammation Fit?. Curr Cardiol Rep 19, 84 (2017). https://doi.org/10.1007/s11886-017-0896-0
Published:
DOI: https://doi.org/10.1007/s11886-017-0896-0