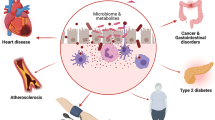
Abstract
Gut microbiota has been recently established to have a contributory role in the development of cardiometabolic disorders, such as atherosclerosis, obesity, and type 2 diabetes. Growing interest has focused on the modulation of gut microbiota as a therapeutic strategy in cardiovascular diseases and metabolic disorders. In this paper, we have reviewed the impact of gut microbiota on metabolic disorders and cardiovascular disease risk, focusing on the newest findings in this field.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Go AS, Mozaffarian D, Roger VL, et al. American heart association statistics committee and stroke statistics subcommittee. Executive summary: heart disease and stroke statistics—2014 update: a report from the American heart association. Circulation. 2014;129(3):399–410. This study provides the most recent updates on the cardiovascular diseases prevalence all over the world.
Ozaki K, Tanaka T. Molecular genetics of coronary artery disease. J Hum Genet. 2015. doi:10.1038/jhg.2015.70.
Kotsis V, Nilsson P, Grassi G, et al. New developments in the pathogenesis of obesity-induced hypertension. J Hypertens. 2015;33(8):1499–508.
Rajilić-Stojanović M, Heilig HG, Tims S, et al. Long-term monitoring of the human intestinal microbiota composition. Lancet. 2010;376(9750):1393–400.
Palm NW, de Zoete MR, Flavell RA. Immune-microbiota interactions in health and disease. Clin Immunol. 2015;159(2):122–7. doi:10.1016/j.clim.2015.05.014. This review explains on the importance of individual gut microbes and on their link with individual disease susceptibility.
Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9.
Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7.
Chen J, Li Y, Tian Y, et al. Interaction between microbes and host intestinal health: modulation by dietary nutrients and gut-brain-endocrine-immune axis. Curr Protein Pept Sci. 2015;16(7):592–603.
De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6.
Mika A, Van Treuren W, González A, et al. Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile versus adult male F344 rats. PLoS ONE. 2015;10(5), e0125889.
Pacheco AR, Sperandio V. Enteric pathogens exploit the microbiota-generated nutritional environment of the gut. Microbiol Spectr. 2015;3(3). This paper gives an interesting overview of microbiota and nutrient generation in the gut.
Kawamoto S, Tran TH, Maruya M, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336(6080):485–9.
Reinhardt C, Bergentall M, Greiner TU, et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature. 2012;483(7391):627–31.
Akira TKS. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84.
Marietta E, Rishi A, Taneja V. Immunogenetic control of the intestinal microbiota. Immunology. 2015;145(3):313–22. This manuscript describes intestinal bacteria enterotypes, and focuses on the genetic factors that influence the composition of the intestinal microflora.
Venkatesh M, Mukherjee S, Wang H, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41(2):296–310. This study provides a description of how a chemical communication between the intestinal symbionts and PXR regulates mucosal integrity through a pathway that involves signaling by TLR4.
Velloso LA, Folli F, Saad MJ. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev. 2015;36(3):245–71.
Zhang M, Chekan JR, Dodd D, et al. Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes. Proc Natl Acad Sci U S A. 2014;111(35):E3708–17.
Kotzampassi K, Giamarellos-Bourboulis E, Stavrou G. Obesity as a consequence of gut bacteria and diet interactions. ISRN Obes. 2014;2014, 651895.
Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5.
Kim MH, Kang SG, Park JH, et al. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145(2):396–406.e1-10. This paper describes the regulation of the immune response by SCFAs and their receptors in the intestines of mice. The pathways described mediate protective immunity and tissue inflammation and may have applications in the clinical practice.
Sina C, Gavrilova O, Förster M, et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183(11):7514–22.
Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829.
Kasubuchi M, Hasegawa S, Hiramatsu T, et al. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–49.
De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. Underlying mechanisms by which soluble dietary fibers promote benefits on body weight and glucose control are poorly understood. This papers describes the most recent evidence in the field.
Li T, Chiang JY. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31(2):159–65.
Fiorucci S, Mencarelli A, Palladino G, et al. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30(11):570–80.
Prawitt J, Caron S, Staels B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr Diabetes Rep. 2011;11(3):160–6.
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77.
Balmer ML, Slack E, de Gottardi A, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6(237), 237ra66. This paper interestingly describes how the liver may act as a functional vascular firewall clearing commensals that have penetrated intestinal circuits. Describes mechanisms may have new therapeutic applications.
Sanz Y, Rastmanesh R, Agostoni C. Understanding the role of gut microbes and probiotics in obesity: how far are we. Pharmacol Res. 2013;69(1):144–55.
Mathur R, Barlow GM. Obesity and the microbiome. Expert Rev Gastroenterol Hepatol. 2015;16:1–13.
Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57.
Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–70.
Bäckhed F, Manchester JK, Semenkovich CF, et al. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104(3):979–84.
Bradlow HL. Obesity and the gut microbiome: pathophysiological aspects. Horm Mol Biol Clin Investig. 2014;17(1):53–61.
Chen J, He X, Huang J. Diet effects in gut microbiome and obesity. Food Sci. 2014;79(4):R442–51.
Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev. 2015;73 Suppl 1:32–40.
Moya-Pérez A, Neef A, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS ONE. 2015;10(7), e0126976. This paper shows mechanisms through which Bifidobacterium pseudocatenulatum reduces systemic inflammation and improves metabolic dysfunction in obese mice. This finding may have a clinical application in the treatment of obesity.
Schleinitz D, Böttcher Y, Blüher M, et al. The genetics of fat distribution. Diabetologia. 2014;57(7):1276–86.
Kong LC, Tap J, Aron-Wisnewsky J, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98(1):16–24.
Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81.
Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015;39(3):198–203.
Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72.
Clarke TB, Davis KM, Lysenko ES, et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16(2):228–31.
Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–31.
Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi:10.1038/nature11450.
Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. This paper show the possibility of a metagenome fingerprint for T2D.
Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5(2), e9085.
Shin NR, Lee JC, Lee HY, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–35. This paper support the hypothesis that the modulation of gut microbiota may increase the antidiabetic effects of metformin.
Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–71. This Authors explore the mechanisms of bacterial regulation of the cross-talk between the host and gut microbiota and provide data on metabolic function of gut bacteria (i.e., A. muciniphila).
Derrien M, Vaughan EE, Plugge CM, et al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(Pt 5):1469–76.
Everard A, Lazarevic V, Derrien M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–86.
Karlsson CL, Onnerfält J, Xu J, et al. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity. 2012;20(11):2257–61.
Hansen CH, Krych L, Nielsen DS, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55(8):2285–94.
Wiedermann CJ, Kiechl S, Dunzendorfer S, et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck study. J Am Coll Cardiol. 1999;34:1975–81.
Miller MA, McTernan PG, Harte AL, et al. Ethnic and sex differences in circulating endotoxin levels: a novel marker of atherosclerotic and cardiovascular risk in a British multi-ethnic population. Atherosclerosis. 2009;203:494–502.
Cappuccio FP, Oakeshott P, Strazzullo P, et al. Application of Framingham risk estimates to ethnic minorities in United Kingdom and implications for primary prevention of heart disease in general practice: cross-sectional population based study. BMJ. 2002;325:1271.
Wożakowska-Kapłon B, Włosowicz M, Gorczyca-Michta I, et al. Oral health status and the occurrence and clinical course of myocardial infarction in hospital phase: a case–control study. Cardiol J. 2013;20(4):370–7. doi:10.5603/CJ.2013.0095.
Holmes E, Loo RL, Stamler J, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453(7193):396–400.
Koren O, Spor A, Felin J, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4592–8.
Karlsson FH, Fåk F, Nookaew I, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245.
Andraws R, Berger JS, Brown DL. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials. JAMA. 2005;293(21):2641–7.
Grayston JT, Kronmal RA, Jackson LA, et al. ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352(16):1637–45.
Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63.
Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–20.
Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103(33):12511–6.
Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63.
Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. Evidence of link between production of TMAO and intestinal microbiota. The TMAO levels are associated with increased risk of major cardiovascular events.
Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35(14):904–10.
Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–14.
Sharma V, Aggarwal A. Helicobacter pylori: does it add to risk of coronary artery disease. World J Cardiol. 2015;7(1):19–25.
Tamer GS, Tengiz I, Ercan E, et al. Helicobacter pylori seropositivity in patients with acute coronary syndromes. Dig Dis Sci. 2009;54:1253–6.
Cangemi R, Casciaro M, Rossi E, et al. Platelet activation is associated with myocardial infarction in patients with pneumonia. J Am Coll Cardiol. 2014;64(18):1917–25.
de Lemos JA, Zirlik A, Schönbeck U, et al. Associations between soluble CD40 ligand, atherosclerosis risk factors, and subclinical atherosclerosis: results from the Dallas Heart Study. Arterioscler Thromb Vasc Biol. 2005;25(10):2192–6.
Furman MI, Barnard MR, Krueger LA, et al. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38(4):1002–6.
Freedman JE, Loscalzo J. Platelet-monocyte aggregates: bridging thrombosis and inflammation. Circulation. 2002;105(18):2130–2.
Miele L, Marrone G, Lauritano C, et al. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19(29):5314–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Luca Miele, Valentina Giorgio, Maria Adele Alberelli, Erica De Candia, Antonio Gasbarrini, and Antonio Grieco declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Diabetes and Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Miele, L., Giorgio, V., Alberelli, M.A. et al. Impact of Gut Microbiota on Obesity, Diabetes, and Cardiovascular Disease Risk. Curr Cardiol Rep 17, 120 (2015). https://doi.org/10.1007/s11886-015-0671-z
Published:
DOI: https://doi.org/10.1007/s11886-015-0671-z