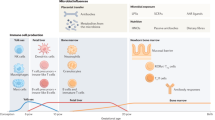
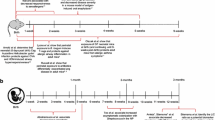
Abstract
Purpose of Review
Allergy and asthma are growing problems in the developed world. The accelerated increase of these diseases may be related to microbiome modification that leads to aberrant activation of Toll-like receptors (TLRs). Current research supports the concept that changes in microbial communities in early life impact TLR activation, resulting in an altered risk for the development of asthma and allergies.
Recent Findings
Prenatal and early childhood events that generate microbiome modification are closely related with TLR activation. Early childhood exposure to a rich array of TLR agonists, particularly lipopolysaccharide, strongly predicts protection against allergic disease later in life even when other lifestyle factors are accounted for. Genetic deletion of TLR signaling components in mice results in reduced function of tolerogenic cell populations in the gut. In contrast, weak TLR signaling can promote allergic sensitization later in life.
Summary
This review summarizes the role of TLR signaling in microbiome-mediated protection against allergy.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
CDC. Allergies. 2017. http://www.cdc.gov/healthcommunication/toolstemplates/entertainmented/tips/Allergies.html.
Begin P, Nadeau KC. Epigenetic regulation of asthma and allergic disease. Allergy Asthma Clin Immunol. 2014;10(1):27. https://doi.org/10.1186/1710-1492-10-27.
Palmer LJ, Burton PR, Faux JA, James AL, Musk AW, Cookson WO. Independent inheritance of serum immunoglobulin E concentrations and airway responsiveness. Am J Respir Crit Care Med. 2000;161(6):1836–43. https://doi.org/10.1164/ajrccm.161.6.9805104.
Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35. https://doi.org/10.1186/s13223-015-0102-0.
van Ree R, Hummelshoj L, Plantinga M, Poulsen LK, Swindle E. Allergic sensitization: host-immune factors. Clin Transl Allergy. 2014;4(1):12. https://doi.org/10.1186/2045-7022-4-12.
Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15(4):410–6. https://doi.org/10.1038/nm.1946.
Gangloff SC, Guenounou M. Toll-like receptors and immune response in allergic disease. Clin Rev Allergy Immunol. 2004;26(2):115–25. https://doi.org/10.1007/s12016-004-0006-0.
Takeda K, Akira S. Roles of Toll-like receptors in innate immune responses. Genes Cells. 2001;6(9):733–42.
Lau MY, Dharmage SC, Burgess JA, Win AK, Lowe AJ, Lodge C, et al. The interaction between farming/rural environment and TLR2, TLR4, TLR6 and CD14 genetic polymorphisms in relation to early- and late-onset asthma. Sci Rep. 2017;7:43681. https://doi.org/10.1038/srep43681.
Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–6. https://doi.org/10.1126/science.1078231.
Mitchell TC, Hildeman D, Kedl RM, Teague TK, Schaefer BC, White J, et al. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat Immunol. 2001;2(5):397–402. https://doi.org/10.1038/87692.
Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–80. https://doi.org/10.1038/90609.
Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. https://doi.org/10.1126/scitranslmed.3008599.
Prince AL, Ma J, Kannan PS, Alvarez M, Gisslen T, Harris RA, et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol. 2016;214(5):627 e1–e16. https://doi.org/10.1016/j.ajog.2016.01.193.
Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8(1):77. https://doi.org/10.1186/s13073-016-0330-z.
Fonseca W, Lukacs NW, Ptaschinski C. Factors affecting the immunity to respiratory syncytial virus: from epigenetics to microbiome. Front Immunol. 2018;9:226. https://doi.org/10.3389/fimmu.2018.00226.
Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–7. https://doi.org/10.1056/NEJM200005183422007.
Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63(6):587–600. https://doi.org/10.1111/j.1600-0897.2010.00848.x.
Karvonen AM, Hyvarinen A, Gehring U, Korppi M, Doekes G, Riedler J, et al. Exposure to microbial agents in house dust and wheezing, atopic dermatitis and atopic sensitization in early childhood: a birth cohort study in rural areas. Clin Exp Allergy. 2012;42(8):1246–56. https://doi.org/10.1111/j.1365-2222.2012.04002.x.
• Karvonen AM, Hyvarinen A, Rintala H, Korppi M, Taubel M, Doekes G, et al. Quantity and diversity of environmental microbial exposure and development of asthma: a birth cohort study. Allergy. 2014;69(8):1092–101. https://doi.org/10.1111/all.12439 This study demostrated the importance of early life microbial exposure in the development of asthma.
Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol. 2006;117(4):817–23. https://doi.org/10.1016/j.jaci.2005.12.1307.
Rautava S, Kainonen E, Salminen S, Isolauri E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol. 2012;130(6):1355–60. https://doi.org/10.1016/j.jaci.2012.09.003.
Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012;9(10):565–76. https://doi.org/10.1038/nrgastro.2012.144.
•• Conrad ML, Ferstl R, Teich R, Brand S, Blumer N, Yildirim AO, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med. 2009;206(13):2869–77. https://doi.org/10.1084/jem.20090845 This work highlights the importance of prenatal Toll-like receptors activation by microbial exposure and the protective effect on the development of allergies.
Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol. 2014;133(2):551–9. https://doi.org/10.1016/j.jaci.2013.06.034.
Dai J, Liu B, Li Z. Regulatory T cells and Toll-like receptors: what is the missing link? Int Immunopharmacol. 2009;9(5):528–33. https://doi.org/10.1016/j.intimp.2009.01.027.
Schaub B, Liu J, Hoppler S, Haug S, Sattler C, Lluis A, et al. Impairment of T-regulatory cells in cord blood of atopic mothers. J Allergy Clin Immunol. 2008;121(6):1491–9, 9 e1-13. https://doi.org/10.1016/j.jaci.2008.04.010.
• Meng SS, Gao R, Yan BD, Ren J, Wu F, Chen P, et al. Maternal allergic disease history affects childhood allergy development through impairment of neonatal regulatory T-cells. Respir Res. 2016;17(1):114. https://doi.org/10.1186/s12931-016-0430-8. This study found that a history of maternal allergy was associated with altered Treg phenotype in neonates, demonstrating that susceptibility to allergy can begin prenatally.
Rodriguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. https://doi.org/10.3402/mehd.v26.26050.
Levin AM, Sitarik AR, Havstad SL, Fujimura KE, Wegienka G, Cassidy-Bushrow AE, et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci Rep. 2016;6:31775. https://doi.org/10.1038/srep31775.
•• Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375(5):411–21. https://doi.org/10.1056/NEJMoa1508749 This study demonstrated the importance of microbial exposure and the development of allergies in two similar genetic communities.
Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–77. https://doi.org/10.1056/NEJMoa020057.
Vuillermin PJ, Macia L, Nanan R, Tang ML, Collier F, Brix S. The maternal microbiome during pregnancy and allergic disease in the offspring. Semin Immunopathol. 2017;39(6):669–75. https://doi.org/10.1007/s00281-017-0652-y.
Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–5. https://doi.org/10.1038/nature07830.
Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37(5):771–83. https://doi.org/10.1016/j.immuni.2012.10.014.
Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149(7):1578–93. https://doi.org/10.1016/j.cell.2012.04.037.
• Kozakova H, Schwarzer M, Tuckova L, Srutkova D, Czarnowska E, Rosiak I, et al. Colonization of germ-free mice with a mixture of three lactobacillus strains enhances the integrity of gut mucosa and ameliorates allergic sensitization. Cell Mol Immunol. 2016;13(2):251–62. https://doi.org/10.1038/cmi.2015.09 This study clearly demonstrates a cause-and-effect relationship between colonization with protective bacteria and protection against allergy.
Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, et al. Commensal bacteria protect against food allergen sensitization. PNAS. 2014;111(36):13145–50. https://doi.org/10.1073/pnas.1412008111.
Fonseca W, Lucey K, Jang S, Fujimura KE, Rasky A, Ting HA, et al. Lactobacillus johnsonii supplementation attenuates respiratory viral infection via metabolic reprogramming and immune cell modulation. Mucosal Immunol. 2017;10(6):1569–80. https://doi.org/10.1038/mi.2017.13.
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–66. https://doi.org/10.1038/nm.3444.
Bashir MEH, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172(11):6978–87. https://doi.org/10.4049/jimmunol.172.11.6978.
Han D, Walsh Matthew C, Cejas Pedro J, Dang Nicholas N, Kim Youngmi F, Kim J, et al. Dendritic cell expression of the signaling molecule TRAF6 is critical for gut microbiota-dependent immune tolerance. Immunity. 2013;38(6):1211–22. https://doi.org/10.1016/j.immuni.2013.05.012.
• Wang S, Charbonnier L-M, Rivas MN, Georgiev P, Li N, Gerber G, et al. MyD88 adaptor-dependent microbial sensing by regulatory T cells promotes mucosal tolerance and enforces commensalism. Immunity. 2015;43(2):289–303. https://doi.org/10.1016/j.immuni.2015.06.014. This study demonstrated that T-cell intrinsic TLR signaling was required to maintain intestinal homeostasis and prevent inflammation. It provided evidence that a protective microbiome limits inflammation through direct interaction with T cells.
He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26(6):812–26. https://doi.org/10.1016/j.immuni.2007.04.014.
Kawano H, Kayama H, Nakama T, Hashimoto T, Umemoto E, Takeda K. IL-10-producing lung interstitial macrophages prevent neutrophilic asthma. Int Immunol. 2016;28(10):489–501. https://doi.org/10.1093/intimm/dxw012.
Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116(2):485–94. https://doi.org/10.1172/JCI25439.
Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18(10):1076–83. https://doi.org/10.1038/ni.3829.
•• Hacini-Rachinel F, Gomez de Agüero M, Kanjarawi R, Moro-Sibilot L, Le Luduec J-B, Macari C, et al. Intestinal dendritic cell licensing through Toll-like receptor 4 is required for oral tolerance in allergic contact dermatitis. J Allergy Clin Immunol. 2018;141(1):163–70. https://doi.org/10.1016/j.jaci.2017.02.022 This study demonstrates that dendritic cell-intrinsic TLR signaling is necessary to promote tolerance against oral antigens at peripheral sites. It provides mechanistic insights to the breakdown of oral tolerance.
Farache J, Koren I, Milo I, Gurevich I, Kim K-W, Zigmond E, et al. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38(3):581–95. https://doi.org/10.1016/j.immuni.2013.01.009.
Jun JC, Jones MB, Oswald DM, Sim ES, Jonnalagadda AR, Kreisman LSC, et al. T cell-intrinsic TLR2 stimulation promotes IL-10 expression and suppressive activity by CD45RbHi T cells. PLoS One. 2017;12(7):e0180688. https://doi.org/10.1371/journal.pone.0180688.
Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116(7):2022–32. https://doi.org/10.1172/JCI28423.
Pace E, Di Sano C, Ferraro M, Bruno A, Caputo V, Gallina S, et al. Budesonide increases TLR4 and TLR2 expression in Treg lymphocytes of allergic asthmatics. Pulm Pharmacol Ther. 2015;32:93–100. https://doi.org/10.1016/j.pupt.2015.02.003.
•• Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351(6275):858–63. https://doi.org/10.1126/science.aac5560 This study demonstrates that dietary antigens sustain a diverse Treg repertoire in mucosal sites.
Wang S, Villablanca EJ, De Calisto J, Gomes DC, Nguyen DD, Mizoguchi E, et al. MyD88-dependent TLR1/2 signals educate dendritic cells with gut-specific imprinting properties. J Immunol. 2011;187(1):141–50. https://doi.org/10.4049/jimmunol.1003740.
Saliganti V, Kapila R, Sharma R, Kapila S. Feeding probiotic Lactobacillus rhamnosus (MTCC 5897) fermented milk to suckling mothers alleviates ovalbumin-induced allergic sensitisation in mice offspring. Br J Nutr. 2015;114(8):1168–79. https://doi.org/10.1017/S000711451500286X.
Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA. Role of TLR4 in allergic sensitization to food proteins in mice. Allergy. 2006;61(1):64–71. https://doi.org/10.1111/j.1398-9995.2006.01012.x.
Thorburn AN, Tseng H-Y, Donovan C, Hansbro NG, Jarnicki AG, Foster PS, et al. TLR2, TLR4 AND MyD88 mediate allergic airway disease (AAD) and Streptococcus pneumoniae-induced suppression of AAD. PLoS One. 2016;11(6):e0156402. https://doi.org/10.1371/journal.pone.0156402.
Kain HL, Osusky R. Pars plana lensectomy in pediatric cataract. Klin Monatsbl Augenheilkd. 1992;200(5):451–3. https://doi.org/10.1055/s-2008-1045790.
•• McAlees JW, Whitehead GS, Harley ITW, Cappelletti M, Rewerts CL, Holdcroft AM, et al. Distinct Tlr4-expressing cell compartments control neutrophilic and eosinophilic airway inflammation. Mucosal Immunol. 2015;8(4):863–73. https://doi.org/10.1038/mi.2014.117 The TLR4 compartment study demonstrates that the role of TLR4 signaling and airway sensitization is an additive effect. Numerous cells relay sensitization signals through TLR4 that culminate in an allergic response.
Thomas SY, Whitehead GS, Takaku M, Ward JM, Xu X, Nakano K, et al. MyD88-dependent dendritic and epithelial cell crosstalk orchestrates immune responses to allergens. Mucosal Immunol. 2017;11:796–810. https://doi.org/10.1038/mi.2017.84.
• Cho M, Lee J-E, Lim H, Shin H-W, Khalmuratova R, Choi G, et al. Fibrinogen cleavage products and Toll-like receptor 4 promote the generation of programmed cell death 1 ligand 2–positive dendritic cells in allergic asthma. J Allergy Clin Immunol. 2017;142:530–541.e6. https://doi.org/10.1016/j.jaci.2017.09.019. This study provides novel insight on how proteases can promote allergic sensitization. The authors show that proteases can act on endogenous proteins to produce TLR4 ligands.
• Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341(6147):792–6. https://doi.org/10.1126/science.1240342.
• Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10(2):299–306. https://doi.org/10.1038/mi.2016.108 This review discusses, in depth, the role of the respiratory tract microbiome. The authors of this manuscript were instrumental in showing that the respiratory tract is not sterile.
O'Connor GT, Lynch SV, Bloomberg GR, Kattan M, Wood RA, Gergen PJ, et al. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol. 2018;141(4):1468–75. https://doi.org/10.1016/j.jaci.2017.06.040.
Liao SL, Tsai MH, Yao TC, Hua MC, Yeh KW, Chiu CY, et al. Caesarean section is associated with reduced perinatal cytokine response, increased risk of bacterial colonization in the airway, and infantile wheezing. Sci Rep. 2017;7(1):9053. https://doi.org/10.1038/s41598-017-07894-2.
Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–91. https://doi.org/10.1038/nm.4176.
Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38(2):321–31. https://doi.org/10.1016/j.clp.2011.03.008.
Bernasconi E, Pattaroni C, Koutsokera A, Pison C, Kessler R, Benden C, et al. Airway microbiota determines innate cell inflammatory or tissue remodeling profiles in lung transplantation. Am J Respir Crit Care Med. 2016;194(10):1252–63. https://doi.org/10.1164/rccm.201512-2424OC.
Larsen JM, Musavian HS, Butt TM, Ingvorsen C, Thysen AH, Brix S. Chronic obstructive pulmonary disease and asthma-associated Proteobacteria, but not commensal Prevotella spp., promote Toll-like receptor 2-independent lung inflammation and pathology. Immunology. 2015;144(2):333–42. https://doi.org/10.1111/imm.12376.
Remot A, Descamps D, Noordine M-L, Boukadiri A, Mathieu E, Robert V, et al. Bacteria isolated from lung modulate asthma susceptibility in mice. ISME J. 2017;11(5):1061–74. https://doi.org/10.1038/ismej.2016.181.
Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4–dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196(12):1645–51. https://doi.org/10.1084/jem.20021340.
• Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349(6252):1106–10. https://doi.org/10.1126/science.aac6623 The farm dust study shows a novel mechanism by which repeated airway exposure of endotoxin can protect against allergy. Earlier reports have focused on Th1/Th2 polarization and the role of adaptive immunity, rather than tissue-intrinsic effects.
•• Shim J-U, Lee SE, Hwang W, Lee C, Park J-W, Sohn J-H, et al. Flagellin suppresses experimental asthma by generating regulatory dendritic cells and T cells. J Allergy Clin Immunol. 2016;137(2):426–35. https://doi.org/10.1016/j.jaci.2015.07.010 This study clearly demonstrates the importance of dose in determining whether TLR5 signaling is protective, or pathogenic, in allergic airway sensitization.
Lee LM, Ji M, Sinha M, Dong MB, Ren X, Wang Y, et al. Determinants of divergent adaptive immune responses after airway sensitization with ligands of Toll-like receptor 5 or Toll-like receptor 9. PLoS One. 2016;11(12):e0167693. https://doi.org/10.1371/journal.pone.0167693.
Kitzmüller C, Kalser J, Mutschlechner S, Hauser M, Zlabinger GJ, Ferreira F, et al. Fusion proteins of flagellin and the major birch pollen allergen Bet v 1 show enhanced immunogenicity, reduced allergenicity, and intrinsic adjuvanticity. J Allergy Clin Immunol. 2018;141(1):293–9.e6. https://doi.org/10.1016/j.jaci.2017.02.044.
Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blümer N, Mutius EV, et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol. 2007;119(6):1514–21. https://doi.org/10.1016/j.jaci.2007.03.023.
• Stein K, Brand S, Jenckel A, Sigmund A, Chen ZJ, Kirschning CJ, et al. Endosomal recognition of Lactococcus lactis G121 and its RNA by dendritic cells is key to its allergy-protective effects. J Allergy Clin Immunol. 2017;139(2):667–78.e5. https://doi.org/10.1016/j.jaci.2016.06.018. This study demonstrated that maturation of tolerogenic dendritic cells is dependent on TLR signaling.
Sudo N, Aiba Y, Oyama N, Yu XN, Matsunaga M, Koga Y, et al. Dietary nucleic acid and intestinal microbiota synergistically promote a shift in the Th1/Th2 balance toward Th1-skewed immunity. Int Arch Allergy Immunol. 2004;135(2):132–5. https://doi.org/10.1159/000080655.
Carver JD, Pimentel B, Cox WI, Barness LA. Dietary nucleotide effects upon immune function in infants. Pediatrics. 1991;88(2):359–63.
Gauvreau GM, Hessel EM, Boulet LP, Coffman RL, O'Byrne PM. Immunostimulatory sequences regulate interferon-inducible genes but not allergic airway responses. Am J Respir Crit Care Med. 2006;174(1):15–20. https://doi.org/10.1164/rccm.200601-057OC.
Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Muller P, et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39(4):562–70. https://doi.org/10.1111/j.1365-2222.2008.03191.x.
Beeh KM, Kanniess F, Wagner F, Schilder C, Naudts I, Hammann-Haenni A, et al. The novel TLR-9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J Allergy Clin Immunol. 2013;131(3):866–74. https://doi.org/10.1016/j.jaci.2012.12.1561.
Casale TB, Cole J, Beck E, Vogelmeier CF, Willers J, Lassen C, et al. CYT003, a TLR9 agonist, in persistent allergic asthma - a randomized placebo-controlled phase 2b study. Allergy. 2015;70(9):1160–8. https://doi.org/10.1111/all.12663.
Aryan Z, Rezaei N. Toll-like receptors as targets for allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2015;15(6):568–74. https://doi.org/10.1097/ACI.0000000000000212.
Starkhammar M, Kumlien Georen S, Swedin L, Dahlen SE, Adner M, Cardell LO. Intranasal administration of poly(I:C) and LPS in BALB/c mice induces airway hyperresponsiveness and inflammation via different pathways. PLoS One. 2012;7(2):e32110. https://doi.org/10.1371/journal.pone.0032110.
Sel S, Wegmann M, Sel S, Bauer S, Garn H, Alber G, et al. Immunomodulatory effects of viral TLR ligands on experimental asthma depend on the additive effects of IL-12 and IL-10. J Immunol. 2007;178(12):7805–13.
Kaufman EH, Fryer AD, Jacoby DB. Toll-like receptor 7 agonists are potent and rapid bronchodilators in guinea pigs. J Allergy Clin Immunol. 2011;127(2):462–9. https://doi.org/10.1016/j.jaci.2010.10.029.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Basic and Applied Science
Rights and permissions
About this article
Cite this article
Michels, K.R., Lukacs, N.W. & Fonseca, W. TLR Activation and Allergic Disease: Early Life Microbiome and Treatment. Curr Allergy Asthma Rep 18, 61 (2018). https://doi.org/10.1007/s11882-018-0815-5
Published:
DOI: https://doi.org/10.1007/s11882-018-0815-5