Abstract
Bioactive lipids are critical regulators of inflammation. Over the last 75 years, these diverse compounds have emerged as clinically-relevant mediators of allergic disease pathophysiology. Animal and human studies have demonstrated the importance of lipid mediators in the development of asthma, allergic rhinitis, urticaria, anaphylaxis, atopic dermatitis, and food allergy. Lipids are critical participants in cell signaling events which influence key physiologic (bronchoconstriction) and immune phenomena (degranulation, chemotaxis, sensitization). Lipid-mediated cellular mechanisms including: (1) formation of structural support platforms (lipid rafts) for receptor signaling complexes, (2) activation of a diverse family of G-protein coupled receptors, and (3) mediating intracellular signaling cascades by acting as second messengers. Here, we review four classes of bioactive lipids (platelet activating factor, the leukotrienes, the prostanoids, and the sphingolipids) with special emphasis on lipid synthesis pathways and signaling, atopic disease pathology, and the ongoing development of atopy treatments targeting lipid mediator pathways.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–24.
Fernandis AZ, Wenk MR. Membrane lipids as signaling molecules. Curr Opin Lipidol. 2007;18(2):121–8.
Kellaway CH, Trethewie ER. The liberation of a slow-reacting smooth muscle-stimulating substance in anaphylaxis. Q J Exp Physiol Cogn Med Sci. 1940;30(2):121–45.
Kihara Y, Gupta S, Maurya MR, Armando A, Shah I, Quehenberger O, et al. Modeling of eicosanoid fluxes reveals functional coupling between cyclooxygenases and terminal synthases. Biophys J. 2014;106(4):966–75.
Sims K, Haynes CA, Kelly S, Allegood JC, Wang E, Momin A, et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J Biol Chem. 2010;285(49):38568–79.
Koberlin MS, Snijder B, Heinz LX, Baumann CL, Fauster A, Vladimer GI, et al. A conserved circular network of coregulated lipids modulates innate immune responses. Cell. 2015;162(1):170–83.
Benveniste J. Platelet-activating factor, a new mediator of anaphylaxis and immune complex deposition from rabbit and human basophils. Nature. 1974;249(457):581–2.
Palgan K, Bartuzi Z. Platelet activating factor in allergies. Int J Immunopathol Pharmacol. 2015;28(4):584–9.
Snyder F. Platelet-activating factor: the biosynthetic and catabolic enzymes. Biochem J. 1995;305(Pt 3):689–705.
Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit Rev Clin Lab Sci. 2003;40(6):643–72.
Zimmerman GA, McIntyre TM, Prescott SM, Stafforini DM. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med. 2002;30(5 Suppl):S294–301.
Kajiwara N, Sasaki T, Bradding P, Cruse G, Sagara H, Ohmori K, et al. Activation of human mast cells through the platelet-activating factor receptor. J Allergy Clin Immunol. 2010;125(5):1137–45. First report showing that PAF induces histamine release from lung mast cells and blood mast cells but not skin mast cells.
Kato M, Yamaguchi T, Tachibana A, Suzuki M, Izumi T, Maruyama K, et al. An atypical protein kinase C, PKC zeta, regulates human eosinophil effector functions. Immunology. 2005;116(2):193–202.
Ruiz J, Monreal M, Sala H, Roncales J, Fiz JA, Monso E, et al. Effects of inhaled platelet activating factor on bronchial responsiveness in patients with symptomatic and asymptomatic pulmonary embolism. Chest. 1992;102(3):819–23.
Cuss FM, Dixon CM, Barnes PJ. Effects of inhaled platelet activating factor on pulmonary function and bronchial responsiveness in man. Lancet. 1986;2(8500):189–92.
Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22.
Mullol J, Bousquet J, Bachert C, Canonica GW, Gimenez-Arnau A, Kowalski ML, et al. Update on rupatadine in the management of allergic disorders. Allergy. 2015;70 Suppl 100:1–24.
Dyer KD, Percopo CM, Xie Z, Yang Z, Kim JD, Davoine F, et al. Mouse and human eosinophils degranulate in response to platelet-activating factor (PAF) and lysoPAF via a PAF-receptor-independent mechanism: evidence for a novel receptor. J Immunol. 2010;184(11):6327–34.
Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704.
Petersen LJ, Church MK, Skov PS. Platelet-activating factor induces histamine release from human skin mast cells in vivo, which is reduced by local nerve blockade. J Allergy Clin Immunol. 1997;99(5):640–7.
Bae R, Arteaga A, Raj JU, Ibe BO. Albuterol isomers modulate platelet-activating factor synthesis and receptor signaling in human bronchial smooth muscle cells. Int Arch Allergy Immunol. 2012;158(1):18–26.
Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358(1):28–35. Important article establishing link between PAF, PAG-AH activity and anaphylaxis.
Vadas P, Perelman B, Liss G. Platelet-activating factor, histamine, and tryptase levels in human anaphylaxis. J Allergy Clin Immunol. 2013;131(1):144–9.
Srinivasan P, Bahnson BJ. Molecular model of plasma PAF acetylhydrolase-lipoprotein association: insights from the structure. Pharmaceuticals. 2010;3(3):541.
Bossi F, Frossi B, Radillo O, Cugno M, Tedeschi A, Riboldi P, et al. Mast cells are critically involved in serum-mediated vascular leakage in chronic urticaria beyond high-affinity IgE receptor stimulation. Allergy. 2011;66(12):1538–45.
Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet activating factor as a mediator and therapeutic approach in bronchial asthma. Inflammation. 2008;31(2):112–20.
Nettis E, Delle Donne P, Di Leo E, Calogiuri GF, Ferrannini A, Vacca A. Rupatadine for the treatment of urticaria. Expert Opin Pharmacother. 2013;14(13):1807–13.
Gimenez-Arnau A, Izquierdo I, Maurer M. The use of a responder analysis to identify clinically meaningful differences in chronic urticaria patients following placebo-controlled treatment with rupatadine 10 and 20 mg. J Eur Acad Dermatol Venereol. 2009;23(9):1088–91.
Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria: a single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol. 2002;110(3):484–8.
Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch Intern Med. 1998;158(11):1213–20.
Miligkos M, Bannuru RR, Alkofide H, Kher SR, Schmid CH, Balk EM. Leukotriene-receptor antagonists versus placebo in the treatment of asthma in adults and adolescents: a systematic review and meta-analysis. Ann Intern Med. 2015;163(10):756–67. Meta-analysis of 50 clinical trials demonstrates efficacy of LTRAs in the treatment of asthma.
Liu MC, Dube LM, Lancaster J. Acute and chronic effects of a 5-lipoxygenase inhibitor in asthma: a 6-month randomized multicenter trial. Zileuton Study Group. J Allergy Clin Immunol. 1996;98(5 Pt 1):859–71.
Singh D, Cadden P, Hunter M, Pearce Collins L, Perkins M, Pettipher R, et al. Inhibition of the asthmatic allergen challenge response by the CRTH2 antagonist OC000459. Eur Respir J. 2013;41(1):46–52.
Straumann A, Hoesli S, Bussmann C, Stuck M, Perkins M, Collins LP, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68(3):375–85.
Idzko M, Hammad H, van Nimwegen M, Kool M, Muller T, Soullie T, et al. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest. 2006;116(11):2935–44.
Tsuji T, Okuno S, Kuroda A, Hamazaki J, Chikami T, Sakurai S, et al. Therapeutic approach to mite-induced intractable dermatitis using novel immunomodulator FTY720 ointment (fingolimod) in NC/Nga mice. Allergol Int. 2016;65(2):172–9.
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–15. Double-blind clinical trial of an S1P receptor functional antagonist showing superior efficacy to standard therapy in relapsing–remitting multiple sclerosis.
Kleinjan A, van Nimwegen M, Leman K, Hoogsteden HC, Lambrecht BN. Topical treatment targeting sphingosine-1-phosphate and sphingosine lyase abrogates experimental allergic rhinitis in a murine model. Allergy. 2013;68(2):204–12.
Price MM, Oskeritzian CA, Falanga YT, Harikumar KB, Allegood JC, Alvarez SE, et al. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent murine model of allergic asthma. J Allergy Clin Immunol. 2013;131(2):501–11. e1.
Gendron D, Lemay AM, Tremblay C, Lai LJ, Langlois A, Bernatchez E, et al. Treatment with a sphingosine analog after the inception of house dust mite-induced airway inflammation alleviates key features of experimental asthma. Respir Res. 2015;16:7.
Dakhale GN, Shinde AT, Mahatme MS, Hiware SK, Mishra DB, Mukhi JI, et al. Clinical effectiveness and safety of cetirizine versus rupatadine in chronic spontaneous urticaria: a randomized, double-blind, 6-week trial. Int J Dermatol. 2014;53(5):643–9.
Maiti R, Jaida J, Raghavendra BN, Goud P, Ahmed I, Palani A. Rupatadine and levocetirizine in chronic idiopathic urticaria: a comparative study of efficacy and safety. J Drugs Dermatol. 2011;10(12):1444–50.
Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340(3):197–206.
Ducharme FM, Hicks GC. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma. Cochrane Database Syst Rev. 2000;3:CD002314.
Kemp JP, Dockhorn RJ, Shapiro GG, Nguyen HH, Reiss TF, Seidenberg BC, et al. Montelukast once daily inhibits exercise-induced bronchoconstriction in 6- to 14-year-old children with asthma. J Pediatr. 1998;133(3):424–8. Double-blind multi-center cross-over study in children (6–14 years of age) showing montelukast attenuates exercise-induced bronchoconstriction.
Hsieh FH, Lam BK, Penrose JF, Austen KF, Boyce JA. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C(4) synthase expression by interleukin 4. J Exp Med. 2001;193(1):123–33.
Cowburn AS, Holgate ST, Sampson AP. IL-5 increases expression of 5-lipoxygenase-activating protein and translocates 5-lipoxygenase to the nucleus in human blood eosinophils. J Immunol. 1999;163(1):456–65.
Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36(6):689–703.
Samuelsson B, Borgeat P, Hammarstrom S, Murphy RC. Introduction of a nomenclature: leukotrienes. Prostaglandins. 1979;17(6):785–7.
Creticos PS, Peters SP, Adkinson Jr NF, Naclerio RM, Hayes EC, Norman PS, et al. Peptide leukotriene release after antigen challenge in patients sensitive to ragweed. N Engl J Med. 1984;310(25):1626–30.
Shirasaki H, Himi T. Role of cysteinyl leukotrienes in allergic rhinitis. Adv Otorhinolaryngol. 2016;77:40–5.
Griffin M, Weiss JW, Leitch AG, McFadden Jr ER, Corey EJ, Austen KF, et al. Effects of leukotriene D on the airways in asthma. N Engl J Med. 1983;308(8):436–9.
Taylor GW, Taylor I, Black P, Maltby NH, Turner N, Fuller RW, et al. Urinary leukotriene E4 after antigen challenge and in acute asthma and allergic rhinitis. Lancet. 1989;1(8638):584–8.
Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. 2002;347(19):1493–9.
Laidlaw TM, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol Allergy Clin N Am. 2013;33(2):195–210.
Lam BK, Austen KF. Leukotriene C4 synthase: a pivotal enzyme in cellular biosynthesis of the cysteinyl leukotrienes. Prostaglandins Other Lipid Mediat. 2002;68-69:511–20.
Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy, Asthma Immunol Res. 2014;6(4):288–95.
Drazen JM, O’Brien J, Sparrow D, Weiss ST, Martins MA, Israel E, et al. Recovery of leukotriene E4 from the urine of patients with airway obstruction. Am Rev Respir Dis. 1992;146(1):104–8.
Aggarwal S, Moodley YP, Thompson PJ, Misso NL. Prostaglandin E2 and cysteinyl leukotriene concentrations in sputum: association with asthma severity and eosinophilic inflammation. Clin Exp Allergy. 2010;40(1):85–93.
Gaber F, Daham K, Higashi A, Higashi N, Gulich A, Delin I, et al. Increased levels of cysteinyl-leukotrienes in saliva, induced sputum, urine and blood from patients with aspirin-intolerant asthma. Thorax. 2008;63(12):1076–82.
Hui Y, Cheng Y, Smalera I, Jian W, Goldhahn L, Fitzgerald GA, et al. Directed vascular expression of human cysteinyl leukotriene 2 receptor modulates endothelial permeability and systemic blood pressure. Circulation. 2004;110(21):3360–6.
Mellor EA, Frank N, Soler D, Hodge MR, Lora JM, Austen KF, et al. Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: Functional distinction from CysLT1R. Proc Natl Acad Sci U S A. 2003;100(20):11589–93.
Kanaoka Y, Maekawa A, Austen KF. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem. 2013;288(16):10967–72. First study to identify GPR99 as an LTE 4 receptor with nanomolar affinity and a functional role in mediating vascular leak in a murine model.
Shirasaki H, Kanaizumi E, Seki N, Himi T. Leukotriene E4 induces MUC5AC release from human airway epithelial NCI-H292 cells. Allergol Int. 2015;64(2):169–74.
Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol. 1998;30(2):173–8.
Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286(5770):264–5.
Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387(6633):620–4.
Gelfand EW, Dakhama A. CD8+ T lymphocytes and leukotriene B4: novel interactions in the persistence and progression of asthma. J Allergy Clin Immunol. 2006;117(3):577–82.
Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156(3 Pt 1):737–43.
Chaudhuri R, Norris V, Kelly K, Zhu CQ, Ambery C, Lafferty J, et al. Effects of a FLAP inhibitor, GSK2190915, in asthmatics with high sputum neutrophils. Pulm Pharmacol Ther. 2014;27(1):62–9.
Vargaftig BB, Singer M. Leukotrienes mediate murine bronchopulmonary hyperreactivity, inflammation, and part of mucosal metaplasia and tissue injury induced by recombinant murine interleukin-13. Am J Respir Cell Mol Biol. 2003;28(4):410–9.
Shin K, Hwang JJ, Kwon BI, Kheradmand F, Corry DB, Lee SH. Leukotriene enhanced allergic lung inflammation through induction of chemokine production. Clin Exp Med. 2015;15(3):233–44.
Espinosa K, Bosse Y, Stankova J, Rola-Pleszczynski M. CysLT1 receptor upregulation by TGF-beta and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J Allergy Clin Immunol. 2003;111(5):1032–40.
Johnson HG, Chinn RA, Chow AW, Bach MK, Nadel JA. Leukotriene-C4 enhances mucus production from submucosal glands in canine trachea in vivo. Int J Immunopharmacol. 1983;5(5):391–6.
Cai Y, Bjermer L, Halstensen TS. Bronchial mast cells are the dominating LTC4S-expressing cells in aspirin-tolerant asthma. Am J Respir Cell Mol Biol. 2003;29(6):683–93.
Pierzchalska M, Szabo Z, Sanak M, Soja J, Szczeklik A. Deficient prostaglandin E2 production by bronchial fibroblasts of asthmatic patients, with special reference to aspirin-induced asthma. J Allergy Clin Immunol. 2003;111(5):1041–8.
Israel E, Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Shapiro J, et al. The pivotal role of 5-lipoxygenase products in the reaction of aspirin-sensitive asthmatics to aspirin. Am Rev Respir Dis. 1993;148(6 Pt 1):1447–51.
Gong Jr H, Linn WS, Terrell SL, Anderson KR, Clark KW. Anti-inflammatory and lung function effects of montelukast in asthmatic volunteers exposed to sulfur dioxide. Chest. 2001;119(2):402–8.
Kraft M, Cairns CB, Ellison MC, Pak J, Irvin C, Wenzel S. Improvements in distal lung function correlate with asthma symptoms after treatment with oral montelukast. Chest. 2006;130(6):1726–32.
Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
Price D, Musgrave SD, Shepstone L, Hillyer EV, Sims EJ, Gilbert RF, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364(18):1695–707.
Bukstein DA, Luskin AT, Bernstein A. "Real-world" effectiveness of daily controller medicine in children with mild persistent asthma. Ann Allergy Asthma Immunol. 2003;90(5):543–9.
Phipatanakul W, Greene C, Downes SJ, Cronin B, Eller TJ, Schneider LC, et al. Montelukast improves asthma control in asthmatic children maintained on inhaled corticosteroids. Ann Allergy Asthma Immunol. 2003;91(1):49–54.
Price DB, Hernandez D, Magyar P, Fiterman J, Beeh KM, James IG, et al. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211–6.
Henderson Jr WR, Chiang GK, Tien YT, Chi EY. Reversal of allergen-induced airway remodeling by CysLT1 receptor blockade. Am J Respir Crit Care Med. 2006;173(7):718–28.
Philip G, Hustad C, Noonan G, Malice MP, Ezekowitz A, Reiss TF, et al. Reports of suicidality in clinical trials of montelukast. J Allergy Clin Immunol. 2009;124(4):691–6. e6.
Philip G, Hustad CM, Malice MP, Noonan G, Ezekowitz A, Reiss TF, et al. Analysis of behavior-related adverse experiences in clinical trials of montelukast. J Allergy Clin Immunol. 2009;124(4):699–706. e8.
Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103(2):147–66.
McCook A, Sune K. Bergstrom. Lancet. 2004;364(9438):930.
Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D(2) pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131(6):1504–12.
Perry CM, McGavin JK, Culy CR, Ibbotson T. Latanoprost: an update of its use in glaucoma and ocular hypertension. Drugs Aging. 2003;20(8):597–630.
Olson DM, Ammann C. Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour. Front Biosci. 2007;12:1329–43.
Naclerio RM, Meier HL, Kagey-Sobotka A, Adkinson Jr NF, Meyers DA, Norman PS, et al. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1983;128(4):597–602.
Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts 2nd LJ. Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol. 1982;129(4):1627–31.
Shen ZJ, Esnault S, Schinzel A, Borner C, Malter JS. The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax activation. Nat Immunol. 2009;10(3):257–65.
Pienkowski MM, Adkinson Jr NF, Plaut M, Norman PS, Lichtenstein LM. Prostaglandin D2 and histamine during the immediate and the late-phase components of allergic cutaneous responses. J Allergy Clin Immunol. 1988;82(1):95–100.
Zhang S, Wu X, Yu S. Prostaglandin D2 receptor D-type prostanoid receptor 2 mediates eosinophil trafficking into the esophagus. Dis Esophagus. 2014;27(6):601–6.
Sawyer N, Cauchon E, Chateauneuf A, Cruz RP, Nicholson DW, Metters KM, et al. Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br J Pharmacol. 2002;137(8):1163–72.
Mohri I, Kadoyama K, Kanekiyo T, Sato Y, Kagitani-Shimono K, Saito Y, et al. Hematopoietic prostaglandin D synthase and DP1 receptor are selectively upregulated in microglia and astrocytes within senile plaques from human patients and in a mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2007;66(6):469–80.
Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32(3):409–18.
Gervais FG, Cruz RP, Chateauneuf A, Gale S, Sawyer N, Nantel F, et al. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol. 2001;108(6):982–8.
Hammad H, de Heer HJ, Soullie T, Hoogsteden HC, Trottein F, Lambrecht BN. Prostaglandin D2 inhibits airway dendritic cell migration and function in steady state conditions by selective activation of the D prostanoid receptor 1. J Immunol. 2003;171(8):3936–40.
Tanaka K, Hirai H, Takano S, Nakamura M, Nagata K. Effects of prostaglandin D2 on helper T cell functions. Biochem Biophys Res Commun. 2004;316(4):1009–14.
Boie Y, Sawyer N, Slipetz DM, Metters KM, Abramovitz M. Molecular cloning and characterization of the human prostanoid DP receptor. J Biol Chem. 1995;270(32):18910–6.
Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287(5460):2013–7.
Wright DH, Ford-Hutchinson AW, Chadee K, Metters KM. The human prostanoid DP receptor stimulates mucin secretion in LS174T cells. Br J Pharmacol. 2000;131(8):1537–45.
Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193(2):255–61.
Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162(3):1278–86.
Mitson-Salazar A, Yin Y, Wansley DL, Young M, Bolan H, Arceo S, et al. Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human TH2 cell subpopulation with enhanced function. J Allergy Clin Immunol. 2016;137(3):907–18. e9.
Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–62. This report identifies a human Lin- CD127+ ILC population that is characterized by CRTH2 expression and present in the lung, gut, nasal tissue and peripheral blood.
Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–8.
Hall IP, Fowler AV, Gupta A, Tetzlaff K, Nivens MC, Sarno M, et al. Efficacy of BI 671800, an oral CRTH2 antagonist, in poorly controlled asthma as sole controller and in the presence of inhaled corticosteroid treatment. Pulm Pharmacol Ther. 2015;32:37–44.
Busse WW, Wenzel SE, Meltzer EO, Kerwin EM, Liu MC, Zhang N, et al. Safety and efficacy of the prostaglandin D2 receptor antagonist AMG 853 in asthmatic patients. J Allergy Clin Immunol. 2013;131(2):339–45.
Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–5.
Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol. 2006;149(6):611–23.
Konya V, Ullen A, Kampitsch N, Theiler A, Philipose S, Parzmair GP, et al. Endothelial E-type prostanoid 4 receptors promote barrier function and inhibit neutrophil trafficking. J Allergy Clin Immunol. 2013;131(2):532–40. Reports that EP4 receptor activation induces pulmonary microvascular endothelial barrier function and suggests EP4 receptors agonists as a potential therapeutic approach for inflammatory diseases.
Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM, Lin PC. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 2006;25(53):7019–28.
Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58(2):362–6.
Peacock CD, Misso NL, Watkins DN, Thompson PJ. PGE 2 and dibutyryl cyclic adenosine monophosphate prolong eosinophil survival in vitro. J Allergy Clin Immunol. 1999;104(1):153–62.
Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2004;25(1):40–6.
Buckley J, Birrell MA, Maher SA, Nials AT, Clarke DL, Belvisi MG. EP4 receptor as a new target for bronchodilator therapy. Thorax. 2011;66(12):1029–35.
Luschnig-Schratl P, Sturm EM, Konya V, Philipose S, Marsche G, Frohlich E, et al. EP4 receptor stimulation down-regulates human eosinophil function. Cell Mol Life Sci. 2011;68(21):3573–87.
Sturm EM, Parzmair GP, Radnai B, Frei RB, Sturm GJ, Hammer A, et al. Phosphoinositide-dependent protein kinase 1 (PDK1) mediates potent inhibitory effects on eosinophils. Eur J Immunol. 2015;45(5):1548–59.
Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone Jr MA, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277(46):44147–54.
Feng C, Beller EM, Bagga S, Boyce JA. Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood. 2006;107(8):3243–50.
Serra-Pages M, Olivera A, Torres R, Picado C, de Mora F, Rivera J. E-prostanoid 2 receptors dampen mast cell degranulation via cAMP/PKA-mediated suppression of IgE-dependent signaling. J Leukoc Biol. 2012;92(6):1155–65.
Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977;1(8001):18–20.
Weksler BB, Marcus AJ, Jaffe EA. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977;74(9):3922–6.
Dusting GJ, Moncada S, Vane JR. Prostacyclin (PGX) is the endogenous metabolite responsible for relaxation of coronary arteries induced by arachindonic acid. Prostaglandins. 1977;13(1):3–15.
Miggin SM, Kinsella BT. Investigation of the mechanisms of G protein: effector coupling by the human and mouse prostacyclin receptors. Identification of critical species-dependent differences. J Biol Chem. 2002;277(30):27053–64.
Vane JR, Botting RM. Pharmacodynamic profile of prostacyclin. Am J Cardiol. 1995;75(3):3A–10A.
Schulman ES, Newball HH, Demers LM, Fitzpatrick FA, Adkinson Jr NF. Anaphylactic release of thromboxane A2, prostaglandin D2, and prostacyclin from human lung parenchyma. Am Rev Respir Dis. 1981;124(4):402–6.
Takahashi Y, Tokuoka S, Masuda T, Hirano Y, Nagao M, Tanaka H, et al. Augmentation of allergic inflammation in prostanoid IP receptor deficient mice. Br J Pharmacol. 2002;137(3):315–22.
Olschewski H. Inhaled iloprost for the treatment of pulmonary hypertension. Eur Respir Rev. 2009;18(111):29–34.
Zhou W, Toki S, Zhang J, Goleniewksa K, Newcomb DC, Cephus JY, et al. Prostaglandin I2 signaling and inhibition of group 2 innate lymphoid cell responses. Am J Respir Crit Care Med. 2016;193(1):31–42.
Safholm J, Manson ML, Bood J, Delin I, Orre AC, Bergman P, et al. Prostaglandin E2 inhibits mast cell-dependent bronchoconstriction in human small airways through the E prostanoid subtype 2 receptor. J Allergy Clin Immunol. 2015;136(5):1232–9. Reports that activation of the EP2 receptor inhibits IgE-dependent contraction of human airways.
Kabashima K, Murata T, Tanaka H, Matsuoka T, Sakata D, Yoshida N, et al. Thromboxane A2 modulates interaction of dendritic cells and T cells and regulates acquired immunity. Nat Immunol. 2003;4(7):694–701.
Thudichum JLW. A treatise on the chemical constitution of the brain. London: Bailliere, Tindall and Cox; 1884.
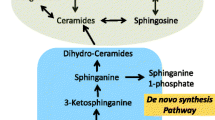
Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5(8):777–82.
Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510(7503):58–67.
Kihara A, Mitsutake S, Mizutani Y, Igarashi Y. Metabolism and biological functions of two phosphorylated sphingolipids, sphingosine 1-phosphate and ceramide 1-phosphate. Prog Lipid Res. 2007;46(2):126–44.
Gangoiti P, Camacho L, Arana L, Ouro A, Granado MH, Brizuela L, et al. Control of metabolism and signaling of simple bioactive sphingolipids: Implications in disease. Prog Lipid Res. 2010;49(4):316–34.
D’Angelo G, Capasso S, Sticco L, Russo D. Glycosphingolipids: synthesis and functions. FEBS J. 2013;280(24):6338–53.
Andreyev AY, Fahy E, Guan Z, Kelly S, Li X, McDonald JG, et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J Lipid Res. 2010;51(9):2785–97.
Surma MA, Klose C, Simons K. Lipid-dependent protein sorting at the trans-Golgi network. Biochim Biophys Acta. 2012;1821(8):1059–67.
Kobayashi T, Mitsuo K, Goto I. Free sphingoid bases in normal murine tissues. Eur J Biochem. 1988;172(3):747–52.
Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, et al. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res. 2010;51(10):3074–87.
Leidl K, Liebisch G, Richter D, Schmitz G. Mass spectrometric analysis of lipid species of human circulating blood cells. Biochim Biophys Acta. 2008;1781(10):655–64.
Vesper H, Schmelz EM, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, Merrill Jr AH. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr. 1999;129(7):1239–50.
Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, et al. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463(7284):1048–53.
Siow DL, Wattenberg BW. Mammalian ORMDL proteins mediate the feedback response in ceramide biosynthesis. J Biol Chem. 2012;287(48):40198–204.
Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316(5822):295–8. Using conditional SphK1/2 knockout animals and bone marrow reconstitution studies, this paper outlines the origins and function of S1P gradients in lymph/plasma, highlighting S1P’s role in lymphocyte trafficking.
Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102(6):669–76.
Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A. 2011;108(23):9613–8.
Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682(1-3):48–55.
Rosen H, Stevens RC, Hanson M, Roberts E, Oldstone MB. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu Rev Biochem. 2013;82:637–62.
Harikumar KB, Yester JW, Surace MJ, Oyeniran C, Price MM, Huang WC, et al. K63-linked polyubiquitination of transcription factor IRF1 is essential for IL-1-induced production of chemokines CXCL10 and CCL5. Nat Immunol. 2014;15(3):231–8.
Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem. 2004;279(12):11320–6. Demonstrates that C1P directly binds, translocates, and activates cPLA2 leading to increased eicosanoid production.
Simanshu DK, Kamlekar RK, Wijesinghe DS, Zou X, Zhai X, Mishra SK, et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature. 2013;500(7463):463–7.
Kulinski JM, Munoz-Cano R, Olivera A. Sphingosine-1-phosphate and other lipid mediators generated by mast cells as critical players in allergy and mast cell function. Eur J Pharmacol. 2016;778:56–67.
Izawa K, Yamanishi Y, Maehara A, Takahashi M, Isobe M, Ito S, et al. The receptor LMIR3 negatively regulates mast cell activation and allergic responses by binding to extracellular ceramide. Immunity. 2012;37(5):827–39.
Izawa K, Isobe M, Matsukawa T, Ito S, Maehara A, Takahashi M, et al. Sphingomyelin and ceramide are physiological ligands for human LMIR3/CD300f, inhibiting FcepsilonRI-mediated mast cell activation. J Allergy Clin Immunol. 2014;133(1):270–3. e1-7.
Wilson BS, Steinberg SL, Liederman K, Pfeiffer JR, Surviladze Z, Zhang J, et al. Markers for detergent-resistant lipid rafts occupy distinct and dynamic domains in native membranes. Mol Biol Cell. 2004;15(6):2580–92.
Zuberbier T, Pfrommer C, Beinholzl J, Hartmann K, Ricklinkat J, Czarnetzki BM. Gangliosides enhance IgE receptor-dependent histamine and LTC4 release from human mast cells. Biochim Biophys Acta. 1995;1269(1):79–84.
Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature. 1996;380(6575):634–6.
Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, et al. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281(5):2515–25.
Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, et al. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199(7):959–70.
Olivera A, Dillahunt SE, Rivera J. Interrogation of sphingosine-1-phosphate receptor 2 function in vivo reveals a prominent role in the recovery from IgE and IgG-mediated anaphylaxis with minimal effect on its onset. Immunol Lett. 2013;150(1-2):89–96.
Dillahunt SE, Sargent JL, Suzuki R, Proia RL, Gilfillan A, Rivera J, et al. Usage of sphingosine kinase isoforms in mast cells is species and/or cell type determined. J Immunol. 2013;190(5):2058–67.
Price MM, Kapitonov D, Allegood J, Milstien S, Oskeritzian CA, Spiegel S. Sphingosine-1-phosphate induces development of functionally mature chymase-expressing human mast cells from hematopoietic progenitors. FASEB J. 2009;23(10):3506–15.
Olivera A, Kitamura Y, Wright LD, Allende ML, Chen W, Kaneko-Goto T, et al. Sphingosine-1-phosphate can promote mast cell hyper-reactivity through regulation of contactin-4 expression. J Leukoc Biol. 2013;94(5):1013–24.
Roviezzo F, Del Galdo F, Abbate G, Bucci M, D’Agostino B, Antunes E, et al. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci U S A. 2004;101(30):11170–5.
Mackle T, Gendy SS, Walsh M, McConn-Walsh R, Costello RW, Walsh MT. Role of sphingosine 1-phosphate receptor expression in eosinophils of patients with allergic rhinitis, and effect of topical nasal steroid treatment on this receptor expression. J Laryngol Otol. 2008;122(12):1309–17.
Moshkovits I, Shik D, Itan M, Karo-Atar D, Bernshtein B, Hershko AY, et al. CMRF35-like molecule 1 (CLM-1) regulates eosinophil homeostasis by suppressing cellular chemotaxis. Mucosal Immunol. 2014;7(2):292–303.
Ha SG, Ge XN, Bahaie NS, Kang BN, Rao A, Rao SP, et al. ORMDL3 promotes eosinophil trafficking and activation via regulation of integrins and CD48. Nat Commun. 2013;4:2479.
Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 2001;15(7):1212–4.
Trifilieff A, Fozard JR. Sphingosine-1-phosphate-induced airway hyper-reactivity in rodents is mediated by the sphingosine-1-phosphate type 3 receptor. J Pharmacol Exp Ther. 2012;342(2):399–406.
Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, et al. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1085–93.
Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448(7152):470–3. First well-powered GWAS study to link a sphingolipid regulatory protein with increased risk for developing a common disease.
Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–21.
Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, Wong KC, et al. A genome-wide association study of global gene expression. Nat Genet. 2007;39(10):1202–7.
Verlaan DJ, Berlivet S, Hunninghake GM, Madore AM, Lariviere M, Moussette S, et al. Allele-specific chromatin remodeling in the ZPBP2/GSDMB/ORMDL3 locus associated with the risk of asthma and autoimmune disease. Am J Hum Genet. 2009;85(3):377–93.
Miller M, Rosenthal P, Beppu A, Mueller JL, Hoffman HM, Tam AB, et al. ORMDL3 transgenic mice have increased airway remodeling and airway responsiveness characteristic of asthma. J Immunol. 2014;192(8):3475–87.
Paulenda T, Draber P. The role of ORMDL proteins, guardians of cellular sphingolipids, in Asthma. Allergy. 2016.
Tibboel J, Reiss I, de Jongste JC, Post M. Sphingolipids in lung growth and repair. Chest. 2014;145(1):120–8.
Petrache I, Natarajan V, Zhen L, Medler TR, Richter A, Berdyshev EV, et al. Ceramide causes pulmonary cell apoptosis and emphysema: a role for sphingolipid homeostasis in the maintenance of alveolar cells. Proc Am Thorac Soc. 2006;3(6):510.
Edukulla R, Liu B, McAlees J, Khurana-Hershey G, Wang Y-H, Lewkowich I, et al. Intratracheal Myriocin enhances allergen-induced TH2 inflammation and airway hyper-responsiveness. Immun Inflamm Dis. 2016.
Petrache I, Kamocki K, Poirier C, Pewzner-Jung Y, Laviad EL, Schweitzer KS, et al. Ceramide synthases expression and role of ceramide synthase-2 in the lung: insight from human lung cells and mouse models. PLoS One. 2013;8(5):e62968.
Diesner SC, Olivera A, Dillahunt S, Schultz C, Watzlawek T, Forster-Waldl E, et al. Sphingosine-kinase 1 and 2 contribute to oral sensitization and effector phase in a mouse model of food allergy. Immunol Lett. 2012;141(2):210–9.
Kurashima Y, Kunisawa J, Higuchi M, Gohda M, Ishikawa I, Takayama N, et al. Sphingosine 1-phosphate-mediated trafficking of pathogenic Th2 and mast cells for the control of food allergy. J Immunol. 2007;179(3):1577–85.
Hamanaka S, Hara M, Nishio H, Otsuka F, Suzuki A, Uchida Y. Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J Invest Dermatol. 2002;119(2):416–23.
Jungersted JM, Agner T. Eczema and ceramides: an update. Contact Dermatitis. 2013;69(2):65–71.
Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96(4):523–6.
Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78(1):27–30.
Miller DW, Koch SB, Yentzer BA, Clark AR, O’Neill JR, Fountain J, et al. An over-the-counter moisturizer is as clinically effective as, and more cost-effective than, prescription barrier creams in the treatment of children with mild-to-moderate atopic dermatitis: a randomized, controlled trial. J Drugs Dermatol. 2011;10(5):531–7.
Jensen JM, Pfeiffer S, Witt M, Brautigam M, Neumann C, Weichenthal M, et al. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2009;123(5):1124–33.
Honda T, Tokura Y, Miyachi Y, Kabashima K. Prostanoid receptors as possible targets for anti-allergic drugs: recent advances in prostanoids on allergy and immunology. Curr Drug Targets. 2010;11(12):1605–13.
Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12(9):688–702.
Acknowledgments
Supported by a Child Health Research Career Development award (NIH K12 HD028827). Special thanks to Shawna Hottinger for her editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Schauberger, Peinhaupt, and Cazares declare no conflicts of interest relevant to this manuscript. Dr. Lindsley declares a Procter Scholarship Career Development Award from Cincinnati Children’s Research Foundation and an JACI Editors Faculty Development Award from the AAAAI Foundation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Basic and Applied Science
Rights and permissions
About this article
Cite this article
Schauberger, E., Peinhaupt, M., Cazares, T. et al. Lipid Mediators of Allergic Disease: Pathways, Treatments, and Emerging Therapeutic Targets. Curr Allergy Asthma Rep 16, 48 (2016). https://doi.org/10.1007/s11882-016-0628-3
Published:
DOI: https://doi.org/10.1007/s11882-016-0628-3