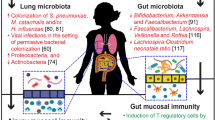
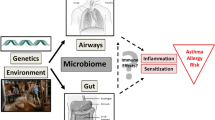
Abstract
Expanding knowledge about an interaction of the bacterial colonization with pathogenic and non-pathogenic bacteria and the human immune system leads to speculation on potential effects on health and disease. Recent advances in sequencing technologies and new bioinformatic possibilities now allow investigating the microbes that colonize the human gut, skin and airways in more detail. In light of the hygiene hypothesis, the impact of the microbial composition of individuals with allergic sensitization and/or atopic diseases, i.e., allergic asthma or atopic eczema, were investigated in several clinical trials. Altered diversity of gut microbiota during infancy as well as colonization with specific pathogenic and apathogenic bacteria has been linked with an elevated risk for allergy. There are ongoing attempts to establish intervention strategies aimed at modifying initial colonization patterns in early life. While results from animal models, in-vitro data and epidemiological studies encourage the concept of a relationship between the microbiome and the development of allergic diseases, the transfer of these findings to intervention strategies still seems to be a major challenge.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Schäfer T, Merkl J, Klemm E, Wichmann HE, Ring J, KORA Study Group. Does my partner cause my allergy? Allergy. 2004;59:781–5.
Hippisley-Cox J, Coupland C, Pringle M, Crown N, Hammersley V. Married couples’ risk of same disease: cross sectional study. Br Med J. 2002;325:636–40.
Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–33.
Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60.
Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992;13:379–81.
Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol. 2010;126:1081–91.
Strachan D. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax. 2000;55:2–10.
Wold AE. The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy. 1998;53:20–5.
Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20.
Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006;6:849–58.
Fouhy F, Ross RP, Fitzgerald G, Stanton C, Cotter PD. Composition of the early intestinal microbiota – Knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes. 2012;3:203–20.
O’Toole PW, Claesson MJ. Gut microbiota: changes throughout the lifespan from infancy to elderly. Int Dairy J. 2010;20:281–91.
Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32:1720–4.
Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–11.
Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24:4–10.
•• The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. In a large population, microbiome samples were collected from healthy volunteers. The authors observed a wide variation of diversity even among healthy subjects.
Dominguez-Bello MG et al. Proc Natl Acad Sci USA. 2010;107:11971–5.
Grönlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19–25.
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21.
Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010;51:77–84.
Roger LC, McCartney AL. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology. 2010;156:3317–28.
Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe. 2011;17:478–82.
•• Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185:385–94. The authors found that formula-fed infants had increased richness of species, with overrepresentation of Clostridium difficile compared with breastfed infants. Escherichia-Shigella and Bacteroides species were underrepresented in infants born by cesarean delivery.
Shen Q, Tuohy KM, Gibson GR, Ward RE. In vitro measurement of the impact of human milk oligosaccharides on the faecal microbiota of weaned formula-fed infants compared to a mixture of prebiotic fructooligosaccharides and galactooligosaccharides. Lett Appl Microbiol. 2011;52:337–43.
Albesharat R, Ehrmann MA, Korakli M, Yazaji S, Vogel RF. Phenotypic and genotypic analyses of lactic acid bacteria in local fermented food, breast milk and faeces of mothers and their babies. Syst Appl Microbiol. 2011;34:148–55.
•• Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Sears MR, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9:15–23. The study population comprised a small sub-sample of 24 healthy, full-term infants from the Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort and provides new evidence that exposure to siblings and/or pets may influence the gut microbiota in early life. The authors speculate that this might have implications for allergic disease in later life.
Schultz M, Gottl C, Young RJ, Iwen P, Vanderhoof JA. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. Journal of Pediatric Gastroenterology and Nutrition. 2004;38(3):293–7.
Gueimonde M, Sakata S, Kalliomaki M, Isolauri E, Benno Y, Salminen S. Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. Journal of Pediatric Gastroenterology and Nutrition. 2006;42(2):166–70.
Renz-Polster H, David MR, Buist AS, Vollmer WM, O'Connor EA, Frazier EA, et al. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005;35:1466–72.
Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedón JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008;122:274–9.
Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010;86:13–5.
•• van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–55. This trial investigated the relationship among microbiota composition, delivery and atopic manifestations at the age of 6–7 years. The authors observed that mode and place of delivery affected the microbiome in the gut, which might subsequently influence the risk of atopic manifestations.
Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–7.
Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–40.
Nylund L, Satokari R, Nikkilä J, Rajilić-Stojanović M, Kalliomäki M, Isolauri E, et al. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children at risk for atopic disease. BMC Microbiol. 2013;13:12.
Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–7.
Brand S, Teich R, Dicke T, Harb H, Yildirim AÖ, Tost J, et al. Epigenetic regulation of in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J Allergy Clin Immunol. 2011;128:618–25.
Schaub B, Liu J, Höppler S, Schleich I, Huehn J, Olek S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. 2009;123:774–82.
•• Pfefferle PI, Prescott SL, Kopp M. Microbial influence on tolerance and opportunities for intervention with prebiotics/probiotics and bacterial lysates. J Allergy Clin Immunol. 2013;131:1453–63. A comprehensive review on the influence of probiotics, prebiotics and other microorganisms on tolerance development and an overview of clinical trials that investigated several intervention strategies.
Grüber C, van Stuijvenberg M, Mosca F, Moro G, Chirico G, Braegger CP, et al. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol. 2010;126:791–7.
Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–9.
Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–71.
Kalliomaki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1019–21.
Kopp MV, Hennemuth I, Dietschek A, Goldstein M, Ihorst G, Heinzmann A, et al. A randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical or immunological effects of Lactobacillus GG supplementation. Pediatrics. 2008;121:e850–6.
Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–8.
Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–91.
Abrahamsson TR, Jakobsson T, Bottcher MF, Fredrikson M, Jenmalm MC, Bjorksten B, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174–80.
Rautava S, Kainonen E, Salminen S, Isolauri E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol. 2012;130:1355–60.
•• Lau S, Gerhold K, Zimmermann K, Ockeloen CW, Rossberg S, Wagner P, et al. Oral application of bacterial lysate in infancy decreases the risk of atopic dermatitis in children with 1 atopic parent in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:1040–7. The trial examines the use of a bacterial lysate. The primary endpoint was negative.
van den Bergh MR, Biesbroek G, Rossen JW, de Steenhuijsen Piters WA, Bosch AA, van Gils EJ, et al. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and bacteria. PLoS One. 2012;7:e47711.
Szabo SM, Levy AR, Gooch KL, Bradt P, Wijaya H, Mitchell I. Elevated risk of asthma after hospitalization for respiratory syncytial virus infection in infancy. Paediatr Respir Rev. 2013;13:9–15.
Ruotsalainen M, Hyvärinen MK, Piippo-Savolainen E, Korppi M. Adolescent asthma after rhinovirus and respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2013;48:633–9.
Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–95.
•• Schwerk N, Brinkmann F, Soudah B, Kabesch M, Hansen G. Wheeze in preschool age is associated with pulmonary bacterial infection and resolves after antibiotic therapy. PLoS One. 2011;6:e27913. Investigating a group of preeschool children with therapy-resistant wheeze, Schwerk showed that chronic bacterial infections are relevant and such children benefit significantly from antibiotic therapy.
Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PloS One. 2010;5:e8578.
De Schutter I, Dreesman A, Soetens O, De Waele M, Crokaert F, Verhaegen J, et al. In young children, persistent wheezing is associated with bronchial bacterial infection: a retrospective analysis. BMC Pediatr. 2012;12:83.
United Nations: World Urbanization Prospects: The 2007 Revision; United Nations, New York, 2008
Compliance with Ethics Guidelines
Conflict of Interest
Meike Bendiks has had travel/accommodations expenses covered/reimbursed by Nestle Infant Nutrition and Abbott GmbH.
Matthias Volkmar Kopp has served on boards for ALK-Abello and Novartis Pharma GmbH; has served as a consultant for Chiesi GmbH, Nestle Nutrition, InfectoPharm, Thermo Fisher Scientific and Bencard GmbH; and has had travel/accommodations expenses covered/reimbursed by Chiesi GmbH.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bendiks, M., Kopp, M.V. The Relationship Between Advances in Understanding the Microbiome and the Maturing Hygiene Hypothesis. Curr Allergy Asthma Rep 13, 487–494 (2013). https://doi.org/10.1007/s11882-013-0382-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11882-013-0382-8