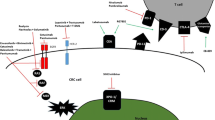
Opinion statement
Standard frontline treatment of metastatic colorectal cancer (CRC) is cytotoxic chemotherapy plus a biologic agent such as an anti-EGFR monoclonal antibody (cetuximab or panitumumab) or anti-VEGF antibody (bevacizumab). Predictive biomarkers include mismatch repair (MMR) status, and RAS and BRAF mutation status; and important factors in treatment selection include primary tumor location, intent of therapy, and potential toxicity, as well as patient age, comorbidities, and patient preference. To date, single-, double-, or triple-agent cytotoxic chemotherapy all have important roles in appropriately selected patients, with the addition of anti-VEGF or anti-EGFR antibody therapy based on the relevant predictive biomarker. Data indicate that patients with proficient MMR, RAS/BRAF wt mCRC are candidates for an anti-EGFR antibody plus doublet chemotherapy if they have a left-sided primary tumor, or for anti-VEGF (bevacizumab) plus doublet or triplet chemotherapy if they have a right-sided primary tumor. Future studies may provide more predictive biomarkers to further personalize therapy for this heterogeneous disease.


Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Society, A.C. Survival rates for colorectal cancer. 2023 [cited 2023 March 4]; Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html.
Siegel RL, et al. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023.
Network, N.C.C. National Comprehensive Cancer Network. Colon Cancer (Version 2.2023). 2023. [cited 2023 May 16]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
Network., N.C.C. National Comprehensive Cancer Network. Rectal Cancer (Version 4.2022). 2022. [cited 2023 March 3]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
Cervantes A, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10–32.
Nordlinger B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–16.
Nordlinger B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–15.
Venderbosch S, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20(20):5322–30.
Le DT, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Le DT, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13.
Antoniotti C, et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022;23(7):876–87.
Elez E, Baraibar I. Immunotherapy in colorectal cancer: an unmet need deserving of change. Lancet Oncol. 2022;23(7):830–1.
Rossini D, et al. FOLFOXIRI plus bevacizumab and atezolizumab as upfront treatment of unresectable metastatic colorectal cancer (mCRC): updated and overall survival results of the phase II randomized AtezoTRIBE study. J Clin Oncol. 2023;41(16_suppl):3500–3500.
Antoniotti C, et al. An immune-related gene expression signature predicts benefit from adding atezolizumab to FOLFOXIRI plus bevacizumab in metastatic colorectal cancer. Clin Cancer Res. 2023;29(12):2291–8.
Andreyev HJ, et al. Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst. 1998;90(9):675–84.
Andreyev HJ, et al. Kirsten ras mutations in patients with colorectal cancer: the “RASCAL II” study. Br J Cancer. 2001;85(5):692–6.
Richman SD, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27(35):5931–7.
Bylsma LC, et al. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: a systematic review and meta-analysis. Cancer Med. 2020;9(3):1044–57.
Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305.
Douillard JY, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Karapetis CS, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–65.
Laurent-Puig P, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27(35):5924–30.
De Roock W, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–62.
Loupakis F, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107(3).
Tejpar S, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194–201.
Cremolini C, et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann Oncol. 2017;28(12):3009–14.
Yoshino T, et al. Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Results from the phase 3 PARADIGM trial. J Clin Oncol. 2022;40(17):LBA1–LBA1.
Watanabe J, et al. Panitumumab vs bevacizumab added to standard first-line chemotherapy and overall survival among patients with RAS wild-type, left-sided metastatic colorectal cancer: a randomized clinical trial. JAMA. 2023;329(15):1271–1282. A large phase 3 randomized clinical trial of doublet chemotherapy plus panitumumab vs bevacizumab in RAS wild-type metastatic CRC, with the primary endpoint of overall survival in patients with left-sided primary tumor location. This is the first clinical trial of anti-EGFR antibody plus chemotherapy to include primary tumor location in primary endpoint.
Shitara K, et al. Negative hyperselection of patients with RAS wild-type metastatic colorectal cancer for panitumumab: a biomarker study of the phase III PARADIGM trial. J Clin Oncol. 2023;41(4_suppl):11–11.
Raghav K, et al. Validation of HER2 amplification as a predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. JCO Precis Oncol. 2019;3:1–13.
Strickler JH, et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): a multicentre, open-label, phase 2 study. Lancet Oncol. 2023;24(5):496–508.
Sartore-Bianchi A, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(6):738–46.
Meric-Bernstam F, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20(4):518–30.
Siena S, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22(6):779–89.
Siena S, et al. Trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-overexpressing/amplified (HER2+) metastatic colorectal cancer (mCRC): primary results from the multicenter, randomized, phase 2 DESTINY-CRC02 study. J Clin Oncol. 2023;41(16_suppl):3501–3501.
Bekaii-Saab TS, et al. MOUNTAINEER-03: Phase 3 study of tucatinib, trastuzumab, and mFOLFOX6 as first-line treatment in HER2+ metastatic colorectal cancer—Trial in progress. J Clin Oncol. 2023;41(4_suppl):TPS261–TPS261.
Douillard JY, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–7.
de Gramont A, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–47.
Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Saltz LB, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–9.
Cunningham D, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45.
Van Cutsem E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17.
Van Cutsem E, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692–700.
Bokemeyer C, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663–71.
Qin S, et al. Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal cancer: the open-label, randomized, phase III TAILOR trial. J Clin Oncol. 2018;36(30):3031–9.
Douillard JY, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–705.
Heinemann V, et al. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br J Cancer. 2021;124(3):587–94.
Heinemann V, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75.
Schwartzberg LS, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32(21):2240–7.
Venook AP, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392–401.
Tol J, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563–72.
Hecht JR, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–80.
Falcone A, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670–6.
Cremolini C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–15.
Loupakis F, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609–18.
Aranda E, et al. FOLFOXIRI plus bevacizumab versus FOLFOX plus bevacizumab for patients with metastatic colorectal cancer and >/=3 circulating tumour cells: the randomised phase III VISNU-1 trial. ESMO Open. 2020;5(6):e000944.
Gruenberger T, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26(4):702–8.
Modest DP, et al. FOLFOXIRI plus panitumumab as first-line treatment of ras wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI study (AIO KRK0109). J Clin Oncol. 2019;37(35):3401–11.
Rossini D, et al. Upfront modified fluorouracil, leucovorin, oxaliplatin, and irinotecan plus panitumumab versus fluorouracil, leucovorin, and oxaliplatin plus panitumumab for patients with RAS/BRAF wild-type metastatic colorectal cancer: the phase III TRIPLETE study by GONO. J Clin Oncol. 2022;40(25): 2878–2888. A phase 3 randomized clinical trial in RAS/BRAF wild-type metastatic colorectal cancer that showed no survival benefit from triplet chemotherapy plus panitumumab compared with doublet chemotherapy plus panitumumab. This was an important trial that showed that if anti EGFR antibody is combined with chemotherapy, doublet chemotherapy is sufficient.
Cremolini C, et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol. 2020:JCO2001225. A meta-analysis of randomized trials of first-line triplet vs doublet chemotherapy plus bevacizumab in metastatic CRC that showed significantly improved survival outcomes in the triplet arm, at the expense of increased toxicity. An important study that showed the benefit of triplet chemotherapy plus bevacizumab in eligible patients across multiple trials.
Cremolini C, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(4):497–507.
Hamaguchi T, et al. A randomized phase III trial of mFOLFOX7 or CapeOX plus bevacizumab versus 5-FU/l-LV or capecitabine plus bevacizumab as initial therapy in elderly patients with metastatic colorectal cancer: JCOG1018 study (RESPECT). J Clin Oncol. 2022;40(4_suppl):10–10.
Van Cutsem E, et al. First-line trifluridine/tipiracil + bevacizumab in patients with unresectable metastatic colorectal cancer: final survival analysis in the TASCO1 study. Br J Cancer. 2022;126(11):1548–54.
Andre T, et al. Trifluridine-tipiracil plus bevacizumab versus capecitabine plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer ineligible for intensive therapy (SOLSTICE): a randomised, open-label phase 3 study. Lancet Gastroenterol Hepatol. 2023;8(2):133–44.
Holch JW, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98.
Lacouture ME, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(8):1351–7.
Bridgewater JA, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(3):398–411.
Primrose J, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15(6):601–11.
Parseghian CM, et al. Anti-EGFR-resistant clones decay exponentially after progression: implications for anti-EGFR re-challenge. Ann Oncol. 2019;30(2):243–9.
Sartore-Bianchi A, et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med. 2022;28(8):1612–8.
Parseghian CM, et al. Phase 2 study of anti-EGFR rechallenge therapy with panitumumab with or without trametinib in advanced colorectal cancer. J Clin Oncol. 2022;40(16_suppl):3520–3520.
Napolitano S, et al. Panitumumab plus trifluridine-tipiracil as anti-epidermal growth factor receptor rechallenge therapy for refractory RAS wild-type metastatic colorectal cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2023.
Kagawa Y, et al. Plasma RAS dynamics and anti-EGFR rechallenge efficacy in patients with RAS/BRAF wild-type metastatic colorectal cancer: REMARRY and PURSUIT trials. J Clin Oncol. 2022;40(16_suppl):3518–3518.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All other authors have no competing interests to declare that are relevant to the content of this article.
FAS reports consulting/advisory funds from Guardant Health, Roche/Genentech, and patent-related royalties from Roche Diagnostics. JMH reports advisory funds from Bayer, Merck, BeiGene, Incyte, Taiho; and research funding to institution from Merck, Treos Bio, Senhwa pharmaceuticals, Bayer, Incyte, TriOncology, Seattle Genetics, Hutchison MediPharma, Pionyr Immunotherapeutics, Trovogene, G1 Therapeutics, Roche, Pfizer, Cardiff Oncology, Mirati Therapeutics, Xilis Inc.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Emiloju, O.E., Zhu, M., Xie, H. et al. Selecting Optimal First-Line Treatment for Microsatellite Stable and Non-Mutated RAS/BRAF Metastatic Colorectal Cancer. Curr. Treat. Options in Oncol. 24, 1739–1757 (2023). https://doi.org/10.1007/s11864-023-01142-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01142-8