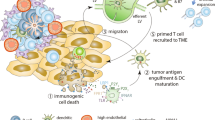
Opinion statement
Merkel cell carcinoma (MCC) has a high risk of recurrence and requires unique treatment relative to other skin cancers. The patient population is generally older, with comorbidities. Multidisciplinary and personalized care is therefore paramount, based on patient preferences regarding risks and benefits. Positron emission tomography and computed tomography (PET-CT) is the most sensitive staging modality and reveals clinically occult disease in ~ 16% of patients. Discovery of occult disease spread markedly alters management. Newly diagnosed, localized disease is often managed with sentinel lymph node biopsy (SLNB), local excision, primary wound closure, and post-operative radiation therapy (PORT). In contrast, metastatic disease is usually treated systemically with an immune checkpoint inhibitor (ICI). However, one or more of these approaches may not be indicated. Criteria for such exceptions and alternative approaches will be discussed. Because MCC recurs in 40% of patients and early detection/treatment of advanced disease is advantageous, close surveillance is recommended. Given that over 90% of initial recurrences arise within 3 years, surveillance frequency can be rapidly decreased after this high-risk period. Patient-specific assessment of risk is important because recurrence risk varies widely (15 to > 80%: Merkelcell.org/recur) depending on baseline patient characteristics and time since treatment. Blood-based surveillance tests are now available (Merkel cell polyomavirus (MCPyV) antibodies and circulating tumor DNA (ctDNA)) with excellent sensitivity that can spare patients from contrast dye, radioactivity, and travel to a cancer imaging facility. If recurrent disease is locoregional, management with surgery and/or RT is typically indicated. ICIs are now the first line for systemic/advanced MCC, with objective response rates (ORRs) exceeding 50%. Cytotoxic chemotherapy is sometimes used for debulking disease or in patients who cannot tolerate ICI. ICI-refractory disease is the major problem faced by this field. Fortunately, numerous promising therapies are on the horizon to address this clinical need.




Similar content being viewed by others
Abbreviations
- ADAM:
-
ADjuvant Avelumab in Merkel
- AE:
-
Adverse effect
- ATTAC:
-
Autologous Transgenic T cells, Avelumab and Class I MHC upregulation for Advanced Checkpoint-inhibitor resistant Merkel cell carcinoma
- CLL:
-
Chronic lymphocytic leukemia
- ctDNA:
-
Circulating tumor DNA
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
- CI:
-
Confidence interval
- CR:
-
Complete response
- CT:
-
Computed tomography
- FGF:
-
Fibroblast growth factor
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- HR:
-
Hazard ratio
- HIV:
-
Human immunodeficiency virus
- ICI:
-
Immune checkpoint inhibition
- irAEs:
-
Immune-related adverse events
- IHC:
-
Immunohistochemical
- IFN-y:
-
Interferon gamma
- IL-12:
-
Interleukin 12
- IT:
-
Intratumoral
- AMERK:
-
MCPyV antibody test
- MCC:
-
Merkel cell carcinoma
- MCPyV:
-
Merkel cell polyomavirus
- MHC:
-
Major histocompatibility complex
- mMCC:
-
Metastatic Merkel cell carcinoma
- NCCN:
-
National Comprehensive Cancer Network
- NED:
-
No evidence of disease
- NET:
-
Neuroendocrine tumors
- OS:
-
Overall survival
- ORR:
-
Overall response rate
- PR:
-
Partial response
- PET-CT:
-
Positron emission tomography and computed tomography
- PFS:
-
Progression-free survival
- PD:
-
Progressive disease
- PDGF:
-
Platelet-derived growth factor
- PRRT:
-
Peptide receptor radionucleotide therapy
- PORT:
-
Post-operative radiation therapy
- PD-(L)1:
-
Programmed cell death (ligand) 1
- RT:
-
Radiation therapy
- RFS:
-
Recurrence-free survival
- SFRT:
-
Single fraction radiation therapy
- SLNB:
-
Sentinel lymph node biopsy
- SSAs:
-
Somatostatin analogs
- SSTR:
-
Somatostatin receptors
- SRS:
-
Somatostatin receptor scintigraphy
- SD:
-
Stable disease
- T-VEC:
-
Talimogene Laherparepvec
- TKIs:
-
Tyrosine kinase inhibitors
- TLR4:
-
Toll-like receptor-4
- TLR9:
-
Toll-like receptor-9
- UV:
-
Ultraviolet
- FDA:
-
US Food and Drug Administration
- VEGF:
-
Vascular endothelial growth factor
- VP:
-
Virus positive
- WES:
-
Whole exome sequencing
- WLE:
-
Wide local excision
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Engels EA, et al. Merkel cell carcinoma and HIV infection. Lancet. 2002;359(9305):497–8. https://doi.org/10.1016/S0140-6736(02)07668-7.
Clarke CA et al. Risk of merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015. 107(2). https://doi.org/10.1093/jnci/dju382.
Heath M, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58(3):375–81. https://doi.org/10.1016/j.jaad.2007.11.020.
Paulson KG, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457-463 e2. https://doi.org/10.1016/j.jaad.2017.10.028.
Harms KL, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23(11):3564–71. https://doi.org/10.1245/s10434-016-5266-4.
•• Singh N, et al. Clinical benefit of baseline imaging in Merkel cell carcinoma: analysis of 584 patients. J Am Acad Dermatol. 2021;84(2):330–9. https://doi.org/10.1016/j.jaad.2020.07.065. This paper is of major importance because baseline imaging detected occult metastatic MCC at a higher rate than reported for melanoma (13.2% vs <1%). Baseline imaging is also indicated for patients with clinically node-negative MCC because upstaging is frequent and markedly alters management and prognosis.
Park SY, et al. How we treat Merkel cell carcinoma: within and beyond current guidelines. Future Oncol. 2021;17(11):1363–77. https://doi.org/10.2217/fon-2020-1036.
Akaike T, Nghiem P. Scientific and clinical developments in Merkel cell carcinoma: a polyomavirus-driven, often-lethal skin cancer. J Dermatol Sci. 2022;105(1):2–10. https://doi.org/10.1016/j.jdermsci.2021.10.004.
Llombart B, et al. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005;46(6):622–34. https://doi.org/10.1111/j.1365-2559.2005.02158.x.
O’Brien S, Berman E, Devetten M. Network NCC NCCN clinical practice guidelines in oncology: chronic myelogenous leukemia. 2010.[(accessed on 3 March 2016)]. Version.
Singh B, et al. Demographics and outcomes of stage I and II Merkel cell carcinoma treated with Mohs micrographic surgery compared with wide local excision in the National Cancer Database. J Am Acad Dermatol. 2018;79(1):126-134 e3. https://doi.org/10.1016/j.jaad.2018.01.041.
Sandel HDt, et al. Merkel cell carcinoma: does tumor size or depth of invasion correlate with recurrence, metastasis, or patient survival? Laryngoscope. 2006;116(5):791–5. https://doi.org/10.1097/01.mlg.0000208615.93883.b2.
Haerle SK, et al. Merkel cell carcinoma of the head and neck: potential histopathologic predictors. Laryngoscope. 2013;123(12):3043–8. https://doi.org/10.1002/lary.24233.
Maloney NJ, et al. Risk factors for and prognostic impact of positive surgical margins after excision of Merkel cell carcinoma. J Am Acad Dermatol. 2022;87(2):444–6. https://doi.org/10.1016/j.jaad.2021.09.014.
Yiengpruksawan A, et al. Merkel cell carcinoma. Prognosis and management. Arch Surg. 1991;126(12):1514–9. https://doi.org/10.1001/archsurg.1991.01410360088014.
Kokoska ER, et al. Early aggressive treatment for Merkel cell carcinoma improves outcome. Am J Surg. 1997;174(6):688–93. https://doi.org/10.1016/s0002-9610(97)00193-1.
Ott MJ, et al. Multimodality management of Merkel cell carcinoma. Arch Surg. 1999;134(4):388–92. https://doi.org/10.1001/archsurg.134.4.388.
Dancey AL, et al. Merkel cell carcinoma: a report of 34 cases and literature review. J Plast Reconstr Aesthet Surg. 2006;59(12):1294–9. https://doi.org/10.1016/j.bjps.2006.03.044.
•• Tarabadkar ES, et al. Narrow excision margins are appropriate for Merkel cell carcinoma when combined with adjuvant radiation: analysis of 188 cases of localized disease and proposed management algorithm. J Am Acad Dermatol. 2021;84(2):340–7. https://doi.org/10.1016/j.jaad.2020.07.079. This paper is of major importance it shows that adjuvant RT-treated patients provided excellent local control rates regardless of surgical margin size. From this cohort, only 1% experienced recurrence in each group (1 of 70 with narrow margins ≤1 cm and 1 of 70 with margins >1 cm; p = .56).
Wang AJ, et al. Merkel cell carcinoma: a forty-year experience at the Peter MacCallum Cancer Centre. BMC Cancer. 2023;23(1):30. https://doi.org/10.1186/s12885-022-10349-1.
Veness M, et al. The role of radiotherapy alone in patients with Merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys. 2010;78(3):703–9.
Santamaria-Barria JA, et al. Merkel cell carcinoma: 30-year experience from a single institution. Ann Surg Oncol. 2013;20(4):1365–73. https://doi.org/10.1245/s10434-012-2779-3.
Gupta SG, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142(6):685–90. https://doi.org/10.1001/archderm.142.6.685.
Ahmadzadehfar H et al. Sensitivity and false negative rate of sentinel lymph node biopsy (SLNB) in malignant melanoma of different parts of the body. 2014. Soc Nuclear Med.
Fang LC, et al. Radiation monotherapy as regional treatment for lymph node-positive Merkel cell carcinoma. Cancer. 2010;116(7):1783–90. https://doi.org/10.1002/cncr.24919.
Leonard JH, et al. Radiation sensitivity of Merkel cell carcinoma cell lines. Int J Radiat Oncol Biol Phys. 1995;32(5):1401–7. https://doi.org/10.1016/0360-3016(94)00610-W.
Lewis KG, et al. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142(6):693–700. https://doi.org/10.1001/archderm.142.6.693.
Harrington C, Kwan W. Radiotherapy and conservative surgery in the locoregional management of Merkel cell carcinoma: the British Columbia Cancer Agency Experience. Ann Surg Oncol. 2016;23(2):573–8. https://doi.org/10.1245/s10434-015-4812-9.
Ghadjar P, et al. The essential role of radiotherapy in the treatment of Merkel cell carcinoma: a study from the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2011;81(4):e583–91. https://doi.org/10.1016/j.ijrobp.2011.05.028.
Strom T, et al. Radiation therapy is associated with improved outcomes in Merkel cell carcinoma. Ann Surg Oncol. 2016;23(11):3572–8. https://doi.org/10.1245/s10434-016-5293-1.
Kang SH, et al. Radiotherapy is associated with significant improvement in local and regional control in Merkel cell carcinoma. Radiat Oncol. 2012;7:171. https://doi.org/10.1186/1748-717X-7-171.
Veness M, et al. The role of radiotherapy alone in patients with merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys. 2010;78(3):703–9. https://doi.org/10.1016/j.ijrobp.2009.08.011.
Hoskin PJ, et al. A prospective randomised trial of 4 Gy or 8 Gy single doses in the treatment of metastatic bone pain. Radiother Oncol. 1992;23(2):74–8. https://doi.org/10.1016/0167-8140(92)90338-u.
Chow E, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–36. https://doi.org/10.1200/JCO.2006.09.5281.
Foro Arnalot P, et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother Oncol. 2008;89(2):150–5. https://doi.org/10.1016/j.radonc.2008.05.018.
Iyer JG, et al. Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med. 2015;4(8):1161–70. https://doi.org/10.1002/cam4.458.
Huynh E, et al. Single-fraction postoperative radiotherapy in early-stage Merkel cell carcioma (MCC): effectiveness and reducedtoxicity. 2023: International Society of Investigative Dermatology, Tokyo, Japan. Abstract #512
•• Cook MM, et al. Postoperative, single-fraction radiation therapy in Merkel cell carcinoma of the head and neck. Adv Radiat Oncol. 2020;5(6):1248–54. https://doi.org/10.1016/j.adro.2020.07.003. This paper is of major importance because it shows that SFRT can offer a potential alternative to conventional post-operative radiation therapy to treat the primary site for localized H&N MCC, particularly in elderly or frail patients, with promising in-field local control and minimal toxicity.
Paulson KG, et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: a prospective validation study. Cancer. 2017;123(8):1464–74. https://doi.org/10.1002/cncr.30475.
Samimi M, et al. Prognostic value of antibodies to Merkel cell polyomavirus T antigens and VP1 protein in patients with Merkel cell carcinoma. Br J Dermatol. 2016;174(4):813–22. https://doi.org/10.1111/bjd.14313.
Lachance K, et al. 590 Detecting Merkel cell carcinoma recurrence using a blood test: outcomes from 774 patients. J Investig Dermatol. 2019;139(5):S101.
Bichakjian CK, et al. Merkel cell carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(6):742–74. https://doi.org/10.6004/jnccn.2018.0055.
Reinert T, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124–31. https://doi.org/10.1001/jamaoncol.2019.0528.
Yeakel J, et al. Bespoke circulating tumor DNA as a biomarker for treatment response in a refractory Merkel cell carcinoma patient. JAAD Case Rep. 2021;18:94–8. https://doi.org/10.1016/j.jdcr.2021.10.025.
Akaike T, HIppe D, So N, Maloney N, Gunnell L, Hall E, Rodriguez A, Aleshin A, Nghiem P, Zaba L. Circulating tumor DNA reflects tumor burdena nd detects early recurrence in patients with Merkel cell carcinoma. 2023: International Society of Investigative Dermatology, Tokyo, Japan. Abstract #1545.
Ramahi E, et al. Merkel cell carcinoma. Am J Clin Oncol. 2013;36(3):299.
•• McEvoy AM, et al. Recurrence and mortality risk of Merkel cell carcinoma by cancer stage and time from diagnosis. JAMA Dermatol. 2022;158(4):382–9. This paper is of major importance because it shows that MCC recurrence rate is approximately 40% which is notably different than that reported for invasive melanoma (approximately 19%), squamous cell carcinoma (approximately 5–9%), or basal cell carcinoma (approximately 1–2%) following definitive therapy. Stage- and time-specific recurrence data can assist in appropriately focusing surveillance resources on patients and time intervals in which recurrence risk is the highest.
Ma JE, Brewer JD. Merkel cell carcinoma in immunosuppressed patients. Cancers (Basel). 2014;6(3):1328–50. https://doi.org/10.3390/cancers6031328.
•• Kim S, et al. Combined nivolumab and ipilimumab with or without stereotactic body radiation therapy for advanced Merkel cell carcinoma: a randomised, open label, phase 2 trial. Lancet. 2022;400(10357):1008–19. https://doi.org/10.1016/S0140-6736(22)01659-2. This paper is of major importance because it illustrates that combined nivolumab and ipilimumab in advanced Merkel cell carcinoma shows clinical benefit in patients with previous anti-PD-1 and PD-L1 treatment and represents a new salvage therapeutic option for advanced MCC.
Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118(1):9–16. https://doi.org/10.1038/bjc.2017.434.
Haanen JB, Robert C. Immune checkpoint inhibitors. Prog. Tumor Res. 2015;42:55–66. https://doi.org/10.1159/000437178.
Kaufman HL, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–85.
D’Angelo SP, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4(9):e180077–e180077.
Nghiem PT, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542–52.
• Nghiem P, et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol. 2019;37(9):693. This work is of importance because it shows pembrolizumab as a generally safe, well tolerated first-line therapy for patients with advanced MCC with a favorable overall survival rates compared to historical data from patients treated with first-line chemotherapy.
FDA grants accelerated approval to retifanlimab-dlwr for metastatic or recurrent locally advanced Merkel cell carcinoma. 2023 03/22/2023 [cited 2023 3/26/2023]; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-retifanlimab-dlwr-metastatic-or-recurrent-locally-advanced-merkel#:~:text=On%20March%2022%2C%202023%2C%20the%20Food%20and%20Drug,carcinoma%20%28MCC%29.%20View%20full%20prescribing%20information%20for%20Zynyz.
LoPiccolo J, et al. Rescue therapy for patients with anti-PD-1-refractory Merkel cell carcinoma: a multicenter, retrospective case series. J Immunother Cancer. 2019;7(1):170. https://doi.org/10.1186/s40425-019-0661-6.
Glutsch V, et al. Activity of ipilimumab plus nivolumab in avelumab-refractory Merkel cell carcinoma. Cancer Immunol Immunother. 2021;70(7):2087–93. https://doi.org/10.1007/s00262-020-02832-0.
Shalhout SZ, et al. A retrospective study of ipilimumab plus nivolumab in anti-PD-L1/PD-1 refractory Merkel cell carcinoma. J Immunother. 2022;45(7):299–302. https://doi.org/10.1097/CJI.0000000000000432.
Khaddour K, et al. Durable remission after rechallenge with ipilimumab and nivolumab in metastatic Merkel cell carcinoma refractory to avelumab: any role for sequential immunotherapy? J Dermatol. 2021;48(2):e80–1. https://doi.org/10.1111/1346-8138.15621.
Appelbaum J, et al. Fatal enteric plexus neuropathy after one dose of ipilimumab plus nivolumab: a case report. J Immunother Cancer. 2018;6(1):82. https://doi.org/10.1186/s40425-018-0396-9.
BAVENCIO® (avelumab) injection, for intravenous use Initial U.S. Approval: 2017. 2017 June 2020 12 Dec 2022]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761049s009lbl.pdf.
KEYTRUDA® (pembrolizumab) injection, for intravenous use Initial U.S. Approval: 2014 2014 March 2021 21 Dec 2022]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf.
Topalian SL et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the CheckMate 358 trial. J Clin Oncol. 2020. JCO2000201. https://doi.org/10.1200/JCO.20.00201.
Bhatia S, et al. ADAM trial: a multicenter, randomized, double-blinded, placebo-controlled, phase 3 trial of adjuvant avelumab (anti-PD-L1 antibody) in merkel cell carcinoma patients with clinically detected lymph node metastases; NCT03271372. J Clin Oncol. 2018;36(15_suppl):TPS9605–TPS9605. https://doi.org/10.1200/JCO.2018.36.15_suppl.TPS9605.
Teke ME, et al. Pembrolizumab compared with standard-of-care observation in treating patients with completely resected stage I-III Merkel cell cancer (STAMP). Ann Surg Oncol. 2022;29(6):3379–80. https://doi.org/10.1245/s10434-022-11498-0.
Becker J, et al. 787O Adjuvant immunotherapy with nivolumab (NIVO) versus observation in completely resected Merkel cell carcinoma (MCC): disease-free survival (DFS) results from ADMEC-O, a randomized, open-label phase II trial. Ann Oncol. 2022;33:S903.
Marchand A, et al. Pembrolizumab and other immune checkpoint inhibitors in locally advanced or metastatic Merkel cell carcinoma: safety and efficacy. Expert Rev Anticancer Ther. 2020;20(12):1093–106.
• Tachiki LM, et al. Impact of duration of immunotherapy on clinical outcomes in advanced Merkel cell carcinoma patients responding to first-line immunotherapy. J Investig Dermatol. 2022;142(10):2840. This work is of importance because patients that continued ICI therapy (median treatment duration: 18 months) experienced higher progression-free survival than those who discontinued treatment (median treatment duration: 12 months). The 2-year progression-free fraction was 80% in the continued treatment group vs 63% in the discontinued group, however this data has not reach statistical significance, HR = 2.11 (95% CI: 0.97–4.57), p=0.059.
Weppler AM, et al. Durability of response to immune checkpoint inhibitors in metastatic Merkel cell carcinoma after treatment cessation. Eur J Cancer. 2023;183:109–18.
Brahmer JR, et al. Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167.
Nghiem P, et al. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol. 2017;13(14):1263–79. https://doi.org/10.2217/fon-2017-0072.
Zhou S, Khanal S, Zhang H. Risk of immune-related adverse events associated with ipilimumab-plus-nivolumab and nivolumab therapy in cancer patients. Ther Clin Risk Manag. 2019;15:211–21. https://doi.org/10.2147/TCRM.S193338.
Barak Y, et al. Regulation of mdm2 expression by p53: alternative promoters produce transcripts with nonidentical translation potential. Genes Dev. 1994;8(15):1739–49. https://doi.org/10.1101/gad.8.15.1739.
Haupt Y, et al. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387(6630):296–9. https://doi.org/10.1038/387296a0.
• Wong MKK, et al. Navtemadlin (KRT-232) activity after failure of anti-PD-1/L1 therapy in patients (pts) with TP53WT Merkel cell carcinoma (MCC). J Clin Oncol. 2022;40(16_suppl):9506–9506. https://doi.org/10.1200/JCO.2022.40.16_suppl.9506. This work is of importance because it highlights navtemadlin, an oral MDM2 inhibitor, as a potential therapeutic option for ICI-refractory MCC.
Veatch J, et al. Merkel polyoma virus specific T-cell receptor transgenic T-cell therapy in PD-1 inhibitor refractory Merkel cell carcinoma. J Clin Oncol. 2022;40(16_suppl):9549–9549. https://doi.org/10.1200/JCO.2022.40.16_suppl.9549.
Knepper TC, et al. An analysis of the use of targeted therapies in patients with advanced Merkel cell carcinoma and an evaluation of genomic correlates of response. Cancer Med. 2021;10(17):5889–96.
Gardair C, et al. Somatostatin receptors 2A and 5 are expressed in Merkel cell carcinoma with no association with disease severity. Neuroendocrinology. 2015;101(3):223–35. https://doi.org/10.1159/000381062.
Caplin ME, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–33. https://doi.org/10.1056/NEJMoa1316158.
Akaike T, et al. High somatostatin receptor expression and efficacy of somatostatin analogues in patients with metastatic Merkel cell carcinoma. Br J Dermatol. 2021;184(2):319–27. https://doi.org/10.1111/bjd.19150.
Basu S, Ranade R. Favorable response of metastatic Merkel cell carcinoma to targeted 177Lu-DOTATATE therapy: will PRRT evolve to become an important approach in receptor-positive cases? J Nucl Med Technol. 2016;44(2):85–7. https://doi.org/10.2967/jnmt.115.163527.
Askari E, et al. Peptide receptor radionuclide therapy in Merkel cell carcinoma: a comprehensive review. J Nucl Med Technol. 2022. https://doi.org/10.2967/jnmt.122.264904.
Ferdinandus J, et al. Response to combined peptide receptor radionuclide therapy and checkpoint immunotherapy with ipilimumab plus nivolumab in metastatic Merkel cell carcinoma. J Nucl Med. 2022;63(3):396–8. https://doi.org/10.2967/jnumed.121.262344.
Sonpavde G, Hutson TE. Pazopanib: a novel multitargeted tyrosine kinase inhibitor. Curr Oncol Rep. 2007;9(2):115–9. https://doi.org/10.1007/s11912-007-0007-2.
Rehman H, et al. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53. https://doi.org/10.1186/s40425-016-0158-5.
Andtbacka RH, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8. https://doi.org/10.1200/JCO.2014.58.3377.
Blackmon JT, et al. Talimogene laherparepvec for regionally advanced Merkel cell carcinoma: A report of 2 cases. JAAD Case Rep. 2017;3(3):185–9. https://doi.org/10.1016/j.jdcr.2017.02.003.
Casale F et al. Complete response of Merkel cell carcinoma with talimogene laherparepvec (TVEC) monotherapy. Dermatol Online J. 2022. 28(1). https://doi.org/10.5070/D328157059.
Knackstedt R, et al. Pre-treated anti-PD-1 refractory Merkel cell carcinoma successfully treated with the combination of PD-1/PD-L1 axis inhibitors and TVEC: a report of two cases. Ann Oncol. 2019;30(8):1399–400. https://doi.org/10.1093/annonc/mdz187.
Westbrook BC, et al. Talimogene laherparepvec induces durable response of regionally advanced Merkel cell carcinoma in 4 consecutive patients. JAAD Case Rep. 2019;5(9):782–6. https://doi.org/10.1016/j.jdcr.2019.06.034.
Bhatia S, et al. Intratumoral G100, a TLR4 agonist, induces antitumor immune responses and tumor regression in patients with Merkel cell carcinoma. Clin Cancer Res. 2019;25(4):1185–95. https://doi.org/10.1158/1078-0432.CCR-18-0469.
Bhatia S, et al. Intratumoral delivery of plasmid IL12 via electroporation leads to regression of injected and noninjected tumors in Merkel cell carcinoma. Clin Cancer Res. 2020;26(3):598–607. https://doi.org/10.1158/1078-0432.CCR-19-0972.
Afanasiev OK, et al. Merkel polyomavirus-specific T cells fluctuate with Merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markersfluctuating and exhausted CD8 T cells in MCC. Clin Cancer Res. 2013;19(19):5351–60.
Pulliam T, et al. LB1029 Correlation of merkel virus-specific CD8 T cells with response to immunotherapy in merkel cell carcinoma. J Investig Dermatol. 2022;142(8):B36.
Ryu H et al. 1045 High dimensional profiling of merkel cell polyomavirus-specific T cells in response to anti-PD-1 immunotherapy. 2022:10;A1085-A1085. https://doi.org/10.1136/jitc-2022-SITC2022.1045.
Church C, et al. Transcriptional and functional analyses of neoantigen-specific CD4 T cells during a profound response to anti-PD-L1 in metastatic Merkel cell carcinoma. J Immunother Cancer. 2022;10(9): e005328.
Funding
Supported in part by the National Institutes of Health/National Cancer Institute, MD, USA, P01 CA225517 and P30 CA015704, the MCC Patient Gift Fund at UW, and Kelsey Dickson Team. Science Courage Research Team Award from the Prostate Cancer Foundation, Santa Monica, CA, USA (Award PCF#19CHAS02). The funding agencies did not participate in the preparation, review, or approval of the manuscript or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Paul Nghiem has served as a consultant for: EMD Serono, Merck, and Pfizer/Regeneron; his institution has received research funding from Bristol-Myers Squibb and EMD Serono. Neha Singh, Erin McClure, Peter Goff, Emily Huynh, Song Park, and Tomoko Akaike declare no conflicts of interest with this work.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, N., McClure, E.M., Akaike, T. et al. The Evolving Treatment Landscape of Merkel Cell Carcinoma. Curr. Treat. Options in Oncol. 24, 1231–1258 (2023). https://doi.org/10.1007/s11864-023-01118-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01118-8