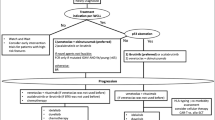
Opinion Statement
Treatment of relapsed and refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) has changed dramatically over the past decade due to the development of oral targeted agents in several therapeutic classes, including BTK inhibitors (such as ibrutinib, acalabrutinib, zanubrutinib, and the non-covalent BTK inhibitor pirtobrutinib), the first in class BCL2 inhibitor venetoclax, PI3K inhibitors (idelalisib and duvelisib), and monoclonal antibodies in monotherapy and in combination. My approach to treatment of the R/R patient draws heavily on prior therapies, such that a patient with no exposure to prior novel therapies would be offered either a BTK or BCL2-based regimen, whereas patients with prior BTK inhibitor exposure would likely receive a BCL2 inhibitor and vice versa. For patients who are intolerant to a BTK inhibitor but are otherwise responding, an alternate BTK inhibitor may be considered. For those patients who have received a fixed-duration BCL2 inhibitor-based regimen and have maintained a response for greater than 12–24 months, re-treatment with a BCL2 inhibitor-based regimen at progression may be considered based on limited data, recognizing that robust prospective clinical trials are lacking in this space. For those patients who are “double refractory” and have progressed on both a BTK inhibitor and a BCL2 inhibitor-based regimen, clinical trials are strongly preferred. In absence of a clinical trial, these patients can be challenged with PI3K inhibitors, though responses are usually not durable, and toxicity is high. Combination cytotoxic chemotherapy with novel agents, allogeneic hematopoietic stem cell transplant, and cellular therapy may be considered for very high-risk populations, such as patients with Richter’s transformation, though novel approaches are urgently needed and clinical trial enrollment is highly encouraged.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mato A, Jahnke J, Li P, et al. Real-world treatment and outcomes among older adults with chronic lymphocytic leukemia before the novel agents era. Haematologica. 2018;103(10):e462–5. https://doi.org/10.3324/haematol.2017.185868.
Surveillance, Epidemiology, and End Results (SEER). SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. Data source(s): SEER Incidence Data, November 2022 Submission (1975-2020), SEER 22 registries. 2023. https://seer.cancer.gov/statistics-network/explorer/. [updated: 2023 Jun 8; cited 2023 Jun 26]
Smolewski P, Robak T. Current treatment of refractory/relapsed chronic lymphocytic leukemia: a focus on novel drugs. Acta Haematologica. 2021;144(4):365–79. https://doi.org/10.1159/000510768.
Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–60. https://doi.org/10.1182/blood-2017-09-806398.
Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals Oncol. 2021;32(1):23–33. https://doi.org/10.1016/j.annonc.2020.09.019.
Woyach JA. Management of relapsed/refractory chronic lymphocytic leukemia. Am J Hematol. 2022;97(S2). https://doi.org/10.1002/ajh.26683.
Crombie J, Davids MS. <i>IGHV</i>mutational status testing in chronic lymphocytic leukemia. Am J Hematol. 2017;92(12):1393–7. https://doi.org/10.1002/ajh.24808.
Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. New England J Med. 2014;371(3):213–23. https://doi.org/10.1056/nejmoa1400376.
• Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–63. https://doi.org/10.1002/ajh.25638. Of import due to the context of this study in establishing long-term safety and durability data for the first novel targeted therapeutic agent avialable in CLL
O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–18. https://doi.org/10.1016/S1470-2045(16)30212-1.
Thompson PA, O'Brien SM, Wierda WG, et al. Complex karyotype is a stronger predictor than del(17p) for an inferior outcome in relapsed or refractory chronic lymphocytic leukemia patients treated with ibrutinib-based regimens. Cancer. 2015;121(20):3612–21. https://doi.org/10.1002/cncr.29566.
Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematol. 2018;103(5):874–9. https://doi.org/10.3324/haematol.2017.182907.
Winqvist M, Andersson P-O, Asklid A, et al. Long-term real-world results of ibrutinib therapy in patients with relapsed or refractory chronic lymphocytic leukemia: 30-month follow up of the Swedish compassionate use cohort. Haematol. 2019;104(5):e208–10. https://doi.org/10.3324/haematol.2018.198820.
Hou JZ, Ryan K, Du S, et al. Real-world ibrutinib dose reductions, holds and discontinuations in chronic lymphocytic leukemia. Future Oncol. 2021;17(35):4959–69. https://doi.org/10.2217/fon-2021-0964.
Lampson BL, Yu L, Glynn RJ, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129(18):2581–4. https://doi.org/10.1182/blood-2016-10-742437.
Boriani G, Menna P, Morgagni R, Minotti G, Vitolo M. Ibrutinib and Bruton’s tyrosine kinase inhibitors in chronic lymphocytic leukemia: focus on atrial fibrillation and ventricular tachyarrhythmias/sudden cardiac death. Chemother. 2022. https://doi.org/10.1159/000528019.
Herman SEM, Montraveta A, Niemann CU, et al. The Bruton tyrosine kinase (BTK) Inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia. Clin Cancer Res. 2017;23(11):2831–41. https://doi.org/10.1158/1078-0432.CCR-16-0463.
• Ghia P, Pluta A, Wach M, et al. Acalabrutinib versus investigator’s choice in relapsed/refractory chronic lymphocytic leukemia: final ASCEND trial results. Hemasphere. 2022;6(12):e801. https://doi.org/10.1097/HS9.0000000000000801. Of importance as the first registrational trial to establish a next-generation BTK inhibitor
•• Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023;388(4):319–32. https://doi.org/10.1056/NEJMoa2211582. Of significant importance as the first head to head clinical trial of BTK inhibitors to establish clinical superiority over ibrutinib.
•• Mato AR, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892–901. https://doi.org/10.1016/S0140-6736(21)00224-5. Of significant importance given the novel non-covalent BTKi mechanism of overcoming BTK resistance muations.
Gomez EB, Ebata K, Randeria HS, et al. Pirtobrutinib preclinical characterization: a highly selective, non-covalent (reversible) BTK inhibitor. Blood. 2023. https://doi.org/10.1182/blood.2022018674.
• Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–52. https://doi.org/10.1200/jco.21.01210. Of importance as the first head to head study of covalent BTKi.
Wang E, Mi X, Thompson MC, et al. Mechanisms of resistance to noncovalent Bruton’s tyrosine kinase inhibitors. N Engl J Med. 2022;386(8):735–43. https://doi.org/10.1056/NEJMoa2114110.
Blombery P, Thompson ER, Lew TE, et al. Enrichment of BTK Leu528Trp mutations in patients with CLL on zanubrutinib: potential for pirtobrutinib cross-resistance. Blood Adv. 2022;6(20):5589–92. https://doi.org/10.1182/bloodadvances.2022008325.
Furman RR, Cheng S, Lu P, et al. Ibrutinib resistance in chronic lymphocytic leukemia. N Engl J Med. 2014;370(24):2352–4. https://doi.org/10.1056/NEJMc1402716.
Gango A, Alpar D, Galik B, et al. Dissection of subclonal evolution by temporal mutation profiling in chronic lymphocytic leukemia patients treated with ibrutinib. Int J Cancer. 2020;146(1):85–93. https://doi.org/10.1002/ijc.32502.
•• Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–22. https://doi.org/10.1056/NEJMoa1513257. Of significant importance establishing viability of BCL2 targeting as an effective treatment strategy in relapsed CLL.
Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol. 2018;36(19):1973–80. https://doi.org/10.1200/JCO.2017.76.6840.
Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768–78. https://doi.org/10.1016/S1470-2045(16)30019-5.
Blombery P, Lew TE, Dengler MA, et al. Clonal hematopoiesis, myeloid disorders and BAX-mutated myelopoiesis in patients receiving venetoclax for CLL. Blood. 2022;139(8):1198–207. https://doi.org/10.1182/blood.2021012775.
•• Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–20. https://doi.org/10.1056/NEJMoa1713976. Of significant importance establishing the definitive venetoclax based treatment regimen in R/R CLL.]
Kater A KT, Eichhorst B, et al. Five-year analysis of Murano study demonstrates enduring undetectable minimal residual disease (uMRD) in a subset of relapsed/refractory chronic lymphocytic leukemia (R/R CLL) patients (pts) following fixed-duration Venetoclax-rituximab (VenR) therapy (Tx) [Abstract 125]. In: Am Soc Hematol Annual Meet 2021. Atlanta, Georgia, USA.
• Seymour JF, Kipps TJ, Eichhorst BF, et al. Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood. 2022;140(8):839–50. https://doi.org/10.1182/blood.2021015014. Of importance in understanding MRD-guided therapy and response duration.
Siddiqui MT, Price A, Ferrajoli A, Borthakur G. Sustained MRD negative remission in del17p and TP53 mutated B cell prolymphocytic leukemia with ibrutinib and venetoclax. Leuk Res Rep. 2021;16:100266. https://doi.org/10.1016/j.lrr.2021.100266.
Lew TE, Anderson MA, Lin VS, et al. Undetectable peripheral blood MRD should be the goal of venetoclax in CLL, but attainment plateaus after 24 months. Blood Adv. 2020;4(1):165–73. https://doi.org/10.1182/bloodadvances.2019000864.
Sharman JP, Coutre SE, Furman RR, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37(16):1391–402. https://doi.org/10.1200/JCO.18.01460.
Thompson PA, Stingo F, Keating MJ, et al. Outcomes of patients with chronic lymphocytic leukemia treated with first-line idelalisib plus rituximab after cessation of treatment for toxicity. Cancer. 2016;122(16):2505–11. https://doi.org/10.1002/cncr.30069.
Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446–55. https://doi.org/10.1182/blood-2018-05-850461.
Meeting ODAC. Phosphatidylinositol 3-Kinase (PI3K) Inhibitors in hematologic malignancies. 2022. https://www.fda.gov/media/157762/download. Accessed May/Jun 2022.
Itchaki G, Brown JR. Lenalidomide in the treatment of chronic lymphocytic leukemia. Expert Opin Investig Drugs. 2017;26(5):633–50. https://doi.org/10.1080/13543784.2017.1313230.
Badoux XC, Keating MJ, Wen S, et al. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2013;31(5):584–91. https://doi.org/10.1200/JCO.2012.42.8623.
Ahn IE, Tian X, Wiestner A. Ibrutinib for chronic lymphocytic leukemia with <i>TP53</i> alterations. New England J Med. 2020;383(5):498–500. https://doi.org/10.1056/nejmc2005943.
Mato AR, Nabhan C, Barr PM, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128(18):2199–205. https://doi.org/10.1182/blood-2016-05-716977.
• Mato AR, Hill BT, Lamanna N, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017;28(5):1050–6. https://doi.org/10.1093/annonc/mdx031. Of importance as a retrospective study to guide sequencing of novel agents.
Davids MS. How should we sequence and combine novel therapies in CLL? Hematology; Am Soc Hematol Educ Program. 2017;1:346–53.
Xu W, Yang S, Zhou K, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;13(1):48. https://doi.org/10.1186/s13045-020-00884-4.
Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–9. https://doi.org/10.1182/blood.2019001160.
Awan FT, Schuh A, Brown JR, et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3(9):1553–62. https://doi.org/10.1182/bloodadvances.2018030007.
Jeffrey Jones MYC, Mato AR, Furman RR, Davids MS, Heffner LT, Cheson BD, Lamanna N, Barr PM, Eradat H, Halwani A, Chyla B, Zhu M, Verdugo M, Humerickhouse RA, Potluri J, Wierda WG, Coutre SE. Venetoclax (VEN) Monotherapy for patients with chronic lymphocytic leukemia (CLL) who relapsed after or were refractory to ibrutinib or idelalisib. Blood. 2016;128(22):637.
Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. The Lancet Oncol. 2018;19(1):65–75. https://doi.org/10.1016/s1470-2045(17)30909-9.
Lin VS, Lew TE, Handunnetti SM, et al. BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax. Blood. 2020;135(25):2266–70. (In eng). https://doi.org/10.1182/blood.2020004782.
Mato AR, Roeker LE, Jacobs R, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020;26(14):3589–96. https://doi.org/10.1158/1078-0432.CCR-19-3815.
Lew TE, Tam CS, Seymour JF. How I treat chronic lymphocytic leukemia after venetoclax. Blood. 2021;138(5):361–9. https://doi.org/10.1182/blood.2020008502.
Thompson MC, Harrup RA, Coombs CC, et al. Venetoclax retreatment of patients with chronic lymphocytic leukemia after a previous venetoclax-based regimen. Blood Adv. 2022;6(15):4553–7. https://doi.org/10.1182/bloodadvances.2022007812.
Scarfò L. Novel therapies and combinations in CLL refractory to BTK inhibitors and venetoclax. Hematol Am Soc Hematol Educ Program. 2022;2022(1):316–22. (In eng). https://doi.org/10.1182/hematology.2022000344.
Lew TE, Lin VS, Cliff ER, et al. Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition. Blood Adv. 2021;5(20):4054–8. (In eng). https://doi.org/10.1182/bloodadvances.2021005083.
Kramer I, Stilgenbauer S, Dietrich S, et al. Allogeneic hematopoietic cell transplantation for high-risk CLL: 10-year follow-up of the GCLLSG CLL3X trial. Blood. 2017;130(12):1477–80. https://doi.org/10.1182/blood-2017-04-775841.
Hillmen P, Rawstron AC, Brock K, et al. Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: the CLARITY study. J Clin Oncol. 2019;37(30):2722–9. https://doi.org/10.1200/JCO.19.00894.
Rogers KA, Huang Y, Ruppert AS, et al. Phase II study of combination obinutuzumab, ibrutinib, and venetoclax in treatment-naive and relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(31):3626–37. https://doi.org/10.1200/JCO.20.00491.
Tam CS, Allan JN, Siddiqi T, et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: primary analysis of the CAPTIVATE FD cohort. Blood. 2022;139(22):3278–89. https://doi.org/10.1182/blood.2021014488.
Kater AP, Owen C, Moreno C, et al. Fixed-duration ibrutinib-venetoclax in patients with chronic lymphocytic leukemia and comorbidities. NEJM Evidence. 2022;1(7). https://doi.org/10.1056/evidoa2200006.
Mato AR, Wierda WG, Ai WZ, et al. NX-2127-001, a First-in-human trial of NX-2127, a Bruton’s tyrosine kinase-targeted protein degrader, in patients with relapsed or refractory chronic lymphocytic leukemia and B-cell malignancies. Blood. 2022;140(Supplement 1):2329–32. https://doi.org/10.1182/blood-2022-164772.
Kater AP, Christensen JH, Bentzen HH, et al. Subcutaneous epcoritamab in patients with relapsed/refractory chronic lymphocytic leukemia: preliminary results from the Epcore CLL-1 trial. Blood. 2021;138(Supplement 1):2627–7. https://doi.org/10.1182/blood-2021-146563.
Budde LE, Assouline S, Sehn LH, et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J Clin Oncol. 2022;40(5):481–91. https://doi.org/10.1200/JCO.21.00931.
Gill SI, Vides V, Frey NV, et al. Prospective clinical trial of anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia shows a high response rate. Blood. 2018;132(Supplement 1):298–8. https://doi.org/10.1182/blood-2018-99-115418.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
PI: Consultant: AbbVie, AstraZeneca, BeiGene, DAVA Oncology, LOXO Oncology; Speaker: Targeted Oncology, The Video Journal of Hematologic Oncology (VJHemOnc).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Islam, P. Current Treatment Options in Relapsed and Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: a Review. Curr. Treat. Options in Oncol. 24, 1259–1273 (2023). https://doi.org/10.1007/s11864-023-01112-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01112-0