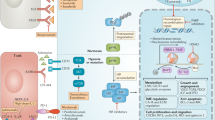
Opinion statement
PRCC is a unique histologic entity compared to other forms of renal cell carcinoma, harboring distinct molecular drivers. The WHO 2022 classification is further emphasizing the molecular biology by making molecular classifications of PRCC subclassifications and discontinuing the morphologic type 1 and type 2 classification system. We agree with this functional classification system and encourage all future clinical trials to only include patients with similar diagnosis instead of conducting basket trials (including all nccRCC together) which limits the scientific value of those conclusions. Based on recent disease-specific clinical trial (S1500, PAPMET), the current standard of care for patients with treatment naïve PRCC is cabozantinib. Prospective clinical trials clearly establish that immune checkpoint inhibitor therapy has meaningful activity in PRCC. The data to date include only single-arm clinical trials of combination immune therapy. Despite the positive and encouraging results, we need validation through randomized studies because of the overestimation of effect size seen in single-arm trials. These randomized trials are currently underway and enrolling. We strongly encourage all physicians to support these studies and enroll patients with PRCC to these trials in order to continue improving the standard of care.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Valenca LB, Hirsch MS, Choueiri TK, Harshman LC. Non-clear cell renal cell carcinoma, part 1: histology. Clin Adv Hematol Oncol. 2015;13(5):308–13.
•• Pal SK, Tangen C, Thompson IM, Jr., Balzer-Haas N, George DJ, Heng DYC, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. 2021;397(10275):695-703. This ransomized clinical trial establishes cabozantinib as the current standard of care for PRCC.
Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17(3):378–88.
Tannir NM, Jonasch E, Albiges L, Altinmakas E, Ng CS, Matin SF, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial. Eur Urol. 2016;69(5):866–74.
Courthod G, Tucci M, Di Maio M, Scagliotti GV. Papillary renal cell carcinoma: a review of the current therapeutic landscape. Crit Rev Oncol Hematol. 2015;96(1):100–12.
•• Williamson SR, Hartmann A, Martignoni G. Papillary renal cell carcinoma. In: WHO Classification of Tumours Editorial Board. Urinary and male genital tumours [Internet]. Lyon (France): International Agency for Research on Cancer; 2022. The updated WHO classification system makes increased focus on molecular sub-classification.
Hawkey CJ. Synthesis of prostaglandin E2, thromboxane B2 and prostaglandin catabolism in gastritis and gastric ulcer. Gut. 1986;27(12):1484–92.
Haake SM, Weyandt JD, Rathmell WK. Insights into the genetic basis of the renal cell carcinomas from The Cancer Genome Atlas. Mol Cancer Res. 2016;14(7):589–98.
Williamson SR, Gill AJ, Argani P, Chen YB, Egevad L, Kristiansen G, et al. Report from the International Society of Urological Pathology (ISUP) Consultation Conference on molecular pathology of urogenital cancers: III: molecular pathology of kidney cancer. Am J Surg Pathol. 2020;44(7):e47-e65.
Trpkov K, Hes O, Williamson SR, Adeniran AJ, Agaimy A, Alaghehbandan R, et al. New developments in existing WHO entities and evolving molecular concepts: the Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod Pathol. 2021;34(7):1392–424.
Cancer Genome Atlas Research N, Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374(2):135–45.
Chen YB, Xu J, Skanderup AJ, Dong Y, Brannon AR, Wang L, et al. Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nat Commun. 2016;7:13131.
Moch HHP, Ulbright TM, Reuter VE. WHO classification of tumors of the urinary system and male genital organs. Lyon: IARC Press; 2016.
Murugan P, Jia L, Dinatale RG, Assel M, Benfante N, Al-Ahmadie HA, et al. Papillary renal cell carcinoma: a single institutional study of 199 cases addressing classification, clinicopathologic and molecular features, and treatment outcome. Mod Pathol. 2022;35(6):825–35.
Lobo J, Ohashi R, Helmchen BM, Rupp NJ, Ruschoff JH, Moch H. The morphological spectrum of papillary renal cell carcinoma and prevalence of provisional/emerging renal tumor entities with papillary growth. Biomedicines. 2021;9(10).
Shen M, Yin X, Bai Y, Zhang H, Ru G, He X, et al. Papillary renal neoplasm with reverse polarity: A CLINICOPATHOLOGICAL and molecular genetic characterization of 16 cases with expanding the morphologic spectrum and further support for a novel entity. Front Oncol. 2022;12: 930296.
Trpkov K, Athanazio D, Magi-Galluzzi C, Yilmaz H, Clouston D, Agaimy A, et al. Biphasic papillary renal cell carcinoma is a rare morphological variant with frequent multifocality: a study of 28 cases. Histopathology. 2018;72(5):777–85.
Przybycin CG, Harper HL, Reynolds JP, Magi-Galluzzi C, Nguyen JK, Wu A, et al. Acquired cystic disease-associated renal cell carcinoma (ACD-RCC): a multiinstitutional study of 40 cases with clinical follow-up. Am J Surg Pathol. 2018;42(9):1156–65.
Aron M, Chang E, Herrera L, Hes O, Hirsch MS, Comperat E, et al. Clear cell-papillary renal cell carcinoma of the kidney not associated with end-stage renal disease: clinicopathologic correlation with expanded immunophenotypic and molecular characterization of a large cohort with emphasis on relationship with renal angiomyoadenomatous tumor. Am J Surg Pathol. 2015;39(7):873–88.
Trpkov K, Abou-Ouf H, Hes O, Lopez JI, Nesi G, Comperat E, et al. Eosinophilic solid and cystic renal cell carcinoma (ESC RCC): further morphologic and molecular characterization of ESC RCC as a distinct entity. Am J Surg Pathol. 2017;41(10):1299–308.
Amin MB, MacLennan GT, Gupta R, Grignon D, Paraf F, Vieillefond A, et al. Tubulocystic carcinoma of the kidney: clinicopathologic analysis of 31 cases of a distinctive rare subtype of renal cell carcinoma. Am J Surg Pathol. 2009;33(3):384–92.
Wang L, Zhang Y, Chen YB, Skala SL, Al-Ahmadie HA, Wang X, et al. VSTM2A overexpression is a sensitive and specific biomarker for mucinous tubular and spindle cell carcinoma (MTSCC) of the kidney. Am J Surg Pathol. 2018;42(12):1571–84.
Argani P, Hicks J, De Marzo AM, Albadine R, Illei PB, Ladanyi M, et al. Xp11 translocation renal cell carcinoma (RCC): extended immunohistochemical profile emphasizing novel RCC markers. Am J Surg Pathol. 2010;34(9):1295–303.
Calio A, Harada S, Brunelli M, Pedron S, Segala D, Portillo SC, et al. TFEB rearranged renal cell carcinoma. A clinicopathologic and molecular study of 13 cases. Tumors harboring MALAT1-TFEB, ACTB-TFEB, and the novel NEAT1-TFEB translocations constantly express PDL1. Mod Pathol. 2021;34(4):842–50.
Kammerer-Jacquet SF, Gandon C, Dugay F, Laguerre B, Peyronnet B, Mathieu R, et al. Comprehensive study of nine novel cases of TFEB-amplified renal cell carcinoma: an aggressive tumour with frequent PDL1 expression. Histopathology. 2022;81(2):228–38.
DiNatale RG, Gorelick AN, Makarov V, Blum KA, Silagy AW, Freeman B, et al. Putative drivers of aggressiveness in TCEB1-mutant renal cell carcinoma: an emerging entity with variable clinical course. Eur Urol Focus. 2021;7(2):381–9.
Ohe C, Smith SC, Sirohi D, Divatia M, de Peralta-Venturina M, Paner GP, et al. Reappraisal of morphologic differences between renal medullary carcinoma, collecting duct carcinoma, and fumarate hydratase-deficient renal cell carcinoma. Am J Surg Pathol. 2018;42(3):279–92.
Kuroda N, Trpkov K, Gao Y, Tretiakova M, Liu YJ, Ulamec M, et al. ALK rearranged renal cell carcinoma (ALK-RCC): a multi-institutional study of twelve cases with identification of novel partner genes CLIP1, KIF5B and KIAA1217. Mod Pathol. 2020;33(12):2564–79.
Swartz MA, Karth J, Schneider DT, Rodriguez R, Beckwith JB, Perlman EJ. Renal medullary carcinoma: clinical, pathologic, immunohistochemical, and genetic analysis with pathogenetic implications. Urology. 2002;60(6):1083–9.
Sirohi DM, Ohe C, Smith SC, Amin MB. SWI/SNF-deficient neoplasms of the genitourinary tract. Semin Diagn Pathol. 2021;38(3):212–21.
Agaimy A, Cheng L, Egevad L, Feyerabend B, Hes O, Keck B, et al. Rhabdoid and undifferentiated phenotype in renal cell carcinoma: analysis of 32 cases indicating a distinctive common pathway of dedifferentiation frequently associated with SWI/SNF complex deficiency. Am J Surg Pathol. 2017;41(2):253–62.
Perret R, Lefort F, Bernhard JC, Baud J, Le Loarer F, Yacoub M. Thyroid-like follicular renal cell carcinoma with sarcomatoid differentiation and an aggressive clinical course: a case report confirming the presence of the EWSR1::PATZ1 fusion gene. Histopathology. 2022;80(4):745–8.
Chumbalkar V, Wang P, Paner GP. Spectrum of biphasic renal cell carcinomas with hyalinized stroma and psammoma bodies associated and not associated with NF2 alteration. Hum Pathol. 2022;121:11–8.
Bersanelli M, Brunelli M, Gnetti L, Maestroni U, Buti S. Pazopanib as a possible option for the treatment of metastatic non-clear cell renal carcinoma patients: a systematic review. Ther Adv Med Oncol. 2020;12:1758835920915303.
Cancel M, Fromont G, Blonz C, Chevreau C, Rioux-Leclercq N, Laguerre B, et al. Everolimus or sunitinib as first-line treatment of metastatic papillary renal cell carcinoma: a retrospective study of the GETUG group (Groupe d’Etude des Tumeurs Uro-Genitales). Eur J Cancer. 2021;158:1–11.
Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26(1):127–31.
Fernandez-Pello S, Hofmann F, Tahbaz R, Marconi L, Lam TB, Albiges L, et al. A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma. Eur Urol. 2017;71(3):426–36.
Ravaud A, Oudard S, De Fromont M, Chevreau C, Gravis G, Zanetta S, et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: a phase II study (SUPAP) by the French Genitourinary Group (GETUG)dagger. Ann Oncol. 2015;26(6):1123–8.
Lee JL, Ahn JH, Lim HY, Park SH, Lee SH, Kim TM, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol. 2012;23(8):2108–14.
Negrier S, Rioux-Leclercq N, Ferlay C, Gross-Goupil M, Gravis G, Geoffrois L, et al. Axitinib in first-line for patients with metastatic papillary renal cell carcinoma: results of the multicentre, open-label, single-arm, phase II AXIPAP trial. Eur J Cancer. 2020;129:107–16.
Bergmann L, Grunwald V, Maute L, Grimm MO, Weikert S, Schleicher J, et al. A randomized phase IIa trial with temsirolimus versus sunitinib in advanced non-clear cell renal cell carcinoma: an intergroup study of the CESAR Central European Society for Anticancer Drug Research-EWIV and the Interdisciplinary Working Group on Renal Cell Cancer (IAGN) of the German Cancer Society. Oncol Res Treat. 2020;43(7–8):333–9.
Choueiri TK, Heng DYC, Lee JL, Cancel M, Verheijen RB, Mellemgaard A, et al. Efficacy of savolitinib vs sunitinib in patients with MET-driven papillary renal cell carcinoma: the SAVOIR phase 3 randomized clinical trial. JAMA Oncol. 2020;6(8):1247–55.
Hutson TE, Michaelson MD, Kuzel TM, Agarwal N, Molina AM, Hsieh JJ, et al. A single-arm, multicenter, phase 2 study of lenvatinib plus everolimus in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. 2021;80(2):162–70.
Feldman DR, Ged Y, Lee CH, Knezevic A, Molina AM, Chen YB, et al. Everolimus plus bevacizumab is an effective first-line treatment for patients with advanced papillary variant renal cell carcinoma: final results from a phase II trial. Cancer. 2020;126(24):5247–55.
Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthelemy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6): e001079.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–41.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–300.
Powles T, Plimack ER, Soulieres D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–73.
• Tachibana H, Kondo T, Ishihara H, Fukuda H, Yoshida K, Takagi T, et al. Modest efficacy of nivolumab plus ipilimumab in patients with papillary renal cell carcinoma. Jpn J Clin Oncol. 2021;51(4):646-53. Prospective study of combination immune therapy.
Koshkin VS, Barata PC, Zhang T, George DJ, Atkins MB, Kelly WJ, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer. 2018;6(1):9.
Gupta R, Ornstein MC, Li H, Allman KD, Wood LS, Gilligan T, et al. Clinical activity of ipilimumab plus nivolumab in patients with metastatic non-clear cell renal cell carcinoma. Clin Genitourin Cancer. 2020;18(6):429–35.
Chahoud J, Msaouel P, Campbell MT, Bathala T, Xiao L, Gao J, et al. Nivolumab for the treatment of patients with metastatic non-clear cell renal cell carcinoma (nccRCC): a single-institutional experience and literature meta-analysis. Oncologist. 2020;25(3):252–8.
Vogelzang NJ, Olsen MR, McFarlane JJ, Arrowsmith E, Bauer TM, Jain RK, et al. Safety and efficacy of nivolumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase IIIb/IV CheckMate 374 study. Clin Genitourin Cancer. 2020;18(6):461–8 e3.
McDermott DF, Lee JL, Bjarnason GA, Larkin JMG, Gafanov RA, Kochenderfer MD, et al. Open-label, single-arm phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced clear cell renal cell carcinoma. J Clin Oncol. 2021;39(9):1020–8.
McDermott DF, Lee JL, Ziobro M, Suarez C, Langiewicz P, Matveev VB, et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol. 2021;39(9):1029–39.
Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18):4156–67.
• Pal SK, McGregor B, Suarez C, Tsao CK, Kelly W, Vaishampayan U, et al. Cabozantinib in combination with atezolizumab for advanced renal cell carcinoma: results from the COSMIC-021 study. J Clin Oncol. 2021;39(33):3725-36. Prospective study of combination immune therapy.
• Lee CH, Voss MH, Carlo MI, Chen YB, Zucker M, Knezevic A, et al. Phase II trial of cabozantinib plus nivolumab in patients with non-clear-cell renal cell carcinoma and genomic correlates. J Clin Oncol. 2022;40(21):2333-41. Prospective study of combination immune therapy.
• Albiges L, Gurney H, Atduev V, Suarez C, Duran MC, David P, et al. Phase 2 KEYNOTE-B61 study of pembrolizumab plus lenvatinib as first-line treatment for non–clear cell renal cell carcinoma. Ann Oncol. 2022;33 (suppl_7):S660-S680. Prospective study of combination immune therapy.
• Powles T, Larkin JMG, Patel P, Pérez-Valderrama B, Rodriguez-Vida A, Glen H, et al. A phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO). J Clin Oncol. 2019;37(suppl 7S; abstr 545). Prospective study of combination immune therapy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
B.L.M. is a paid consultant/advisor to Abbvie, Pfizer, AVEO oncology, Janssen, Astellas, Bristol-Myers Squibb, Clovis, Tempus, Merck, Exelixis, Bayer Oncology, Lilly, and Peloton Therapeutics; Huntsman Cancer Institute has received research funding from Exelixis (Inst), Bavarian-Nordic (Inst), Clovis (Inst), Genentech (Inst), and Bristol-Myers Squibb (Inst) on his behalf. I have received financial compensation as a paid consultant/advisor to Abbvie, Pfizer, AVEO oncology, Janssen, Astellas, Bristol-Myers Squibb, Clovis, Tempus, Merck, Exelixis, Bayer Oncology, Lilly, and Peloton Therapeutics; Huntsman Cancer Institute has received research funding from Exelixis, Bavarian-Nordic, Clovis, and Bristol-Myers Squibb on my behalf.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maughan, B.L., Sirohi, D. Papillary Renal Cell Carcinoma: A Review of Prospective Clinical Trials. Curr. Treat. Options in Oncol. 24, 1199–1212 (2023). https://doi.org/10.1007/s11864-023-01107-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01107-x