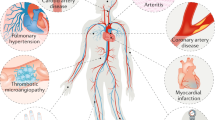
Opinion statement
Vascular events have become an important issue in the overall management of cancer patients. They usually result from a combination of (i) direct or indirect toxicity of anticancer treatments, (ii) a higher prevalence of cardiovascular risk factors in cancer patients, and (iii) prolonged exposure to treatments due to an increasing patient survival rate. In addition to conventional chemotherapies and radiotherapy, targeted therapies and immunotherapies have been developed which improve the prognosis of cancer patients but sometimes at the cost of vascular toxicity, which can lead to systemic or pulmonary hypertension and arterial/venous thromboembolic events. Endothelial dysfunction, a procoagulant state and metabolic disorders are the three main pathophysiological patterns leading to cancer treatment-related vascular toxicity. This issue is challenging because serious vascular adverse events can necessitate cancer treatment being put on hold or stopped, which could compromise patient survival. In addition to increasing the risk of thrombotic adverse events, cancer therapies may lead to an increased risk of bleeding, especially in treatments with vascular endothelial growth factor inhibitors. Therefore, we can define vasculo-oncology as a part of the cardio-oncology specialty; its aims are to predict, prevent, screen, and treat vascular toxicity related to cancer treatments. While the level of evidence is low regarding the management of vascular toxicity during cancer therapy, cardiologists and specialists in vascular diseases should closely collaborate with oncologists and hematologists to determine the optimal strategy for each patient.



Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Grilz E, Königsbrügge O, Posch F, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018;103:1549–56.
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–801.
• Herrmann J, Lerman A. An update on cardio-oncology. Trends Cardiovasc Med. 2014;24:285–95 This reference is of importance because clearly explains physiopathological mechanisms of cardiac and vascular toxicities induced by cancer therapies.
Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies. J Am Coll Cardiol. 2015;66:1160–78.
Cameron AC, Touyz RM, Lang NN. Vascular complications of cancer chemotherapy. Can J Cardiol. 2016;32:852–62.
Grover SP, Hisada YM, Kasthuri RS, Reeves BN, Mackman N. Cancer therapy–associated thrombosis. ATVB. 2021;41:1291–305.
Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–58.
Campia U, Moslehi JJ, Amiri-Kordestani L, et al. Cardio-oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation. 2019;139 Available at: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000641. Accessed August 24, 2021.
Lai E, Cascinu S, Scartozzi M. Are all anti-angiogenic drugs the same in the treatment of second-line metastatic colorectal cancer? Expert Opinion on Clinical Practice. Front Oncol. 2021;11:637823.
Faruque LI, Lin M, Battistella M, et al. Systematic review of the risk of adverse outcomes associated with vascular endothelial growth factor inhibitors for the treatment of cancer. PLoS ONE. 2014;9:e101145.
Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–9.
Ngo DTM, Williams T, Horder S, et al. Factors associated with adverse cardiovascular events in cancer patients treated with Bevacizumab. J Clin Med. 2020;9:E2664.
Versmissen J, Mirabito Colafella KM, Koolen SLW, Danser AHJ. Vascular cardio-oncology: vascular endothelial growth factor inhibitors and hypertension. Cardiovasc Res. 2019;115:904–14.
Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017.
Totzeck M, Mincu RI, Rassaf T. Cardiovascular adverse events in patients with cancer treated with bevacizumab: a meta-analysis of more than 20 000 patients. J Am Heart Assoc. 2017;6:e006278.
Qi W-X, Shen Z, Tang L-N, Yao Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta-analysis. Crit Rev Oncol Hematol. 2014;92:71–82.
Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–30. https://doi.org/10.1056/NEJMoa1406470 Available at: https://www.nejm.org/doi/10.1056/NEJMoa1406470. Accessed October 30, 2021.
Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. JCO. 2013;31:3639–46.
Choueiri TK, Schutz FAB, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–5.
Grothey A, Cutsem EV, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12.
Demetri GD, Reichardt P, Kang Y-K, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302.
Qi W-X, Min D-L, Shen Z, et al. Risk of venous thromboembolic events associated with VEGFR-TKIs: A systematic review and meta-analysis. Int J Cancer. 2013;132:2967–74.
Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–85.
Hedhli N, Russell KS. Cardiotoxicity of molecularly targeted agents. Curr Cardiol Rev. 2011;7:221–33.
Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology. 2010;79:27–38.
Je Y, Schutz FAB, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10:967–74.
Li C-C, Tsai H-L, Huang C-W, Yeh Y-S, Tsai T-H, Wang J-Y. Iatrogenic pseudoaneurysm after bevacizumab therapy in patients with metastatic colorectal cancer: two case reports. Mol Clin Oncol. 2018;9:499–503.
Oshima Y, Tanimoto T, Yuji K, Tojo A. Association between aortic dissection and systemic exposure of vascular endothelial growth factor pathway inhibitors in the Japanese Adverse Drug Event Report Database. Circulation. 2017;135:815–7.
Rix U, Hantschel O, Dürnberger G, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–63.
Weatherald J, Bondeelle L, Chaumais M-C, et al. Pulmonary complications of Bcr-Abl tyrosine kinase inhibitors. Eur Respir J. 2020;56:2000279 Available at: https://erj.ersjournals.com/content/56/4/2000279. Accessed November 1, 2021.
Kantarjian H, Jabbour E, Grimley J, Kirkpatrick P. Dasatinib. Nat Rev Drug Discov. 2006;5:717–8.
Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–88.
Santoro M, Mancuso S, Accurso V, Di Lisi D, Novo G, Siragusa S. Cardiovascular issues in tyrosine kinase inhibitors treatments for chronic myeloid leukemia: a review. Front Physiol. 2021;12:675811.
Haguet H, Douxfils J, Mullier F, Chatelain C, Graux C, Dogné J-M. Risk of arterial and venous occlusive events in chronic myeloid leukemia patients treated with new generation BCR-ABL tyrosine kinase inhibitors: a systematic review and meta-analysis. Expert Opin Drug Saf. 2017;16:5–12.
Hamadi A, Grigg AP, Dobie G, et al. Ponatinib tyrosine kinase inhibitor induces a thromboinflammatory response. Thromb Haemost. 2019;119:1112–23.
Haguet H, Douxfils J, Chatelain C, Graux C, Mullier F, Dogné J-M. BCR-ABL tyrosine kinase inhibitors: which mechanism(s) may explain the risk of thrombosis? TH Open. 2018;2:e68–88.
Aichberger KJ, Herndlhofer S, Schernthaner G-H, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86:533–9.
Mulas O, Caocci G, Mola B, La Nasa G. Arterial hypertension and tyrosine kinase inhibitors in chronic myeloid leukemia: a systematic review and meta-analysis. Front Pharmacol. 2021;12:674748.
Cornet L, Khouri C, Roustit M, et al. Pulmonary arterial hypertension associated with protein kinase inhibitors: a pharmacovigilance–pharmacodynamic study. Eur Respir J. 2019;53:1802472 Available at: https://erj.ersjournals.com/content/53/5/1802472. Accessed November 1, 2021.
Banks M, Crowell K, Proctor A, Jensen BC. Cardiovascular effects of the MEK inhibitor, trametinib: a case report, literature review, and consideration of mechanism. Cardiovasc Toxicol. 2017;17:487–93.
Kubin T, Cetinkaya A, Schönburg M, Beiras-Fernandez A, Walther T, Richter M. The MEK1 inhibitors UO126 and PD98059 block PDGF-AB induced phosphorylation of threonine 292 in porcine smooth muscle cells. Cytokine. 2017;95:51–4.
Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–46. https://doi.org/10.1152/physrev.00054.2009.
Mincu RI, Mahabadi AA, Michel L, et al. Cardiovascular adverse events associated with BRAF and MEK inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2019;2:e198890.
Wémeau M, Gauthier J, Leleu X, Yakoub-Agha I. IMiD en hématologie. Bull Cancer. 2011;98:879–87.
El Accaoui RN, Shamseddeen WA, Taher AT. Thalidomide and thrombosis. A meta-analysis. Thromb Haemost. 2007;97:1031–6.
Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–66.
Li A, Wu Q, Warnick G, et al. The incidence of thromboembolism for lenalidomide versus thalidomide in older patients with newly diagnosed multiple myeloma. Ann Hematol. 2020;99:121–6.
Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20:57–73.
Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012;97:442–50.
Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N Engl J Med. 2015;372:142–52.
Zhai Y, Ye X, Hu F, et al. Cardiovascular Toxicity of Carfilzomib: The real-world evidence based on the adverse event reporting system database of the FDA, the United States. Front Cardiovasc Med. 2021;8:735466.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade Longo DL, editor. N Engl J Med. 2018;378:158–68.
Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. 2018;71:1755–64.
Salem J-E, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–89.
Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol. 2018;37:2579–84.
• Drobni ZD, Alvi RM, Taron J, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–311 This reference is of importance because highlights a new type of cardiac toxicity mediated by immunotherapy with an impact in clinical practice and in the management of patients receiving immunotherapy and presented cardiac toxicity.
Solinas C, Saba L, Sganzerla P, Petrelli F. Venous and arterial thromboembolic events with immune checkpoint inhibitors: A systematic review. Thromb Res. 2020;196:444–53.
Thein KZ, Htut TW, Ball S, Swarup S, Sultan A, Oo TH. Venous thromboembolism risk in patients with hormone receptor-positive HER2-negative metastatic breast cancer treated with combined CDK 4/6 inhibitors plus endocrine therapy versus endocrine therapy alone: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res Treat. 2020;183:479–87.
Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–96.
Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–66.
Hernandez RK, Sørensen HT, Pedersen L, Jacobsen J, Lash TL. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115:4442–9.
Nathan L, Shi W, Dinh H, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci U S A. 2001;98:3589–93.
Khosrow-Khavar F, Filion KB, Bouganim N, Suissa S, Azoulay L. Aromatase inhibitors and the risk of cardiovascular outcomes in women with breast cancer. Circulation. 2020;141:549–59.
Matthews A, Stanway S, Farmer RE, et al. Long term adjuvant endocrine therapy and risk of cardiovascular disease in female breast cancer survivors: systematic review. BMJ. 2018;363:k3845.
Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, Van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol. 2015;68:386–96.
Gheorghe GS, Hodorogea AS, Ciobanu A, Nanea IT, Gheorghe ACD. Androgen deprivation therapy, hypogonadism and cardiovascular toxicity in men with advanced prostate cancer. Curr Oncol. 2021;28:3331–46.
D’Amico AV, Chen M-H, Renshaw A, Loffredo M, Kantoff PW. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2015;314:1291–3.
Morgans AK, Fan K-H, Koyama T, et al. Influence of age on incident diabetes and cardiovascular disease in prostate cancer survivors receiving androgen deprivation therapy. J Urol. 2015;193:1226–31.
Punnen S, Cooperberg MR, Sadetsky N, Carroll PR. Androgen deprivation therapy and cardiovascular risk. J Clin Oncol. 2011;29:3510–6.
Nanda A, Chen M-H, Braccioforte MH, Moran BJ, D’Amico AV. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302:866–73.
O’Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33:1243–51.
D’Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–5.
Alibhai SMH, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–8.
Iacovelli R, Ciccarese C, Bria E, et al. The cardiovascular toxicity of Abiraterone and Enzalutamide in prostate cancer. Clin Genitourin Cancer. 2018;16:e645–53.
Proverbs-Singh T, Chiu SK, Liu Z, et al. Arterial thromboembolism in cancer patients treated with cisplatin: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1837–40.
Teoh JY-C, Tian X-Y, Wong CY-P, et al. Endothelial dysfunction after androgen deprivation therapy and the possible underlying mechanisms. Prostate. 2022;82:13–25.
Shore ND, Saad F, Cookson MS, et al. Oral Relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187–96.
Challa AA, Calaway AC, Cullen J, et al. Cardiovascular Toxicities of Androgen Deprivation Therapy. Curr Treat Options in Oncol. 2021;22:47.
•• Lyon AR, Dent S, Stanway S, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22:1945–60 This reference is of outstanding importance because provides a step-by-step approach and evidence-based risk stratification tools to stratify oncological patients receiving therapies known to have cardio and vasculo-toxicities.
•• Alexandre J, Cautela J, Ederhy S, et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European Cardio-Oncology Guidelines. JAHA. 2020;9:e018403 Available at: https://www.ahajournals.org/doi/10.1161/JAHA.120.018403. Accessed November 2, 2021. This reference is of outstanding importance because provides a pragmatic and synthetic approach in the management of patients receiving cancer therapies with potential cardiovascular toxicities.
•• Herrmann J, Lenihan D, Armenian S, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–99 This reference is of outstanding importance because updates management of cardiovascular toxicities in patients receiving cancer therapies according to recent data and literature.
Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648–71.
•• Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566–81 This reference is of outstanding importance because updates guidelines in the treatment and prophylaxis of venous thromboembolism in patients with cancer.
Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Meilhac, A., Cautela, J. & Thuny, F. Cancer Therapies and Vascular Toxicities. Curr. Treat. Options in Oncol. 23, 333–347 (2022). https://doi.org/10.1007/s11864-022-00964-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-022-00964-2