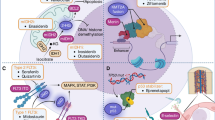
Opinion statement
Cytogenetics and mutation identification in acute myeloid leukemia have allowed for more targeted therapy. Many therapies have been approved by the FDA in the last 3 years including gilteritinib and azacitidine but the overall survival has remained stagnant at 25%. The inability to achieve complete remission was related to the residual leukemic stem cells (LSCs). Thus, the relationship between bone marrow niche and LSCs must be further explored to prevent treatment relapse/resistance. The development of immunotherapy and nanotechnology may play a role in future therapy to achieve the complete remission. Nano-encapsulation of drugs can improve drugs’ bioavailability, help drugs evade resistance, and provide combination therapy directly to the cancer cells. Studies indicate targeting surface antigens such as CLL1 and CD123 using chimeric antibody receptor T cells can improve survival outcomes. Finally, new discoveries indicate that inhibiting integrin αvβ3 and acid ceramidase may prove to be efficacious.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J Hematol Oncol. 2019;12(1):100.
Wunderlich M, Mizukawa B, Chou FS, Sexton C, Shrestha M, Saunthararajah Y, et al. AML cells are differentially sensitive to chemotherapy treatment in a human xenograft model. Blood. 2013;121(12):e90–7.
Ossenkoppele G, Schuurhuis GJ. MRD in AML: does it already guide therapy decision-making? Hematol Am Soc Hematol Educ Program. 2016;2016(1):356–65.
Chen XHX, Wood BL, Walter RB, Pagel JM, Becker PS, et al. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol. 2015;33:1258–64.
Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17(9):1086–93.
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.
Short NJ, Kantarjian H, Ravandi F, Daver N. Emerging treatment paradigms with FLT3 inhibitors in acute myeloid leukemia. Ther Adv Hematol. 2019;10:2040620719827310.
Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124(23):3441–9.
Sami SA, Darwish NHE, Barile ANM, Mousa SA. Current and future molecular targets for acute myeloid leukemia therapy. Curr Treat Options in Oncol. 2020;21(1):3.
Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299–312.
Stone RM. The addition of midostaurin to standard chemotherapy decreases cumulative incidence of relapse (CIR) in the International Prospective Randomized, Placebo-Controlled, Double-Blind Trial (CALGB 10603 / RATIFY [Alliance]) for newly diagnosed acute myeloid leukemia (AML) patients with FLT3 mutations. 2017.
Sung PJ, Sugita M, Koblish H, Perl AE, Carroll M. Hematopoietic cytokines mediate resistance to targeted therapy in FLT3-ITD acute myeloid leukemia. Blood Adv. 2019;3(7):1061–72.
Dumas PY, Naudin C, Martin-Lanneree S, Izac B, Casetti L, Mansier O, et al. Hematopoietic niche drives FLT3-ITD acute myeloid leukemia resistance to quizartinib via STAT5-and hypoxia-dependent upregulation of AXL. Haematologica. 2019;104(10):2017–27.
Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol. 2017;18(8):1061–75.
James AJ, Smith CC, Litzow M, Perl AE, Altman JK, Shepard D, et al. Pharmacokinetic profile of gilteritinib: a novel FLT-3 tyrosine kinase inhibitor. Clin Pharmacokinet. 2020.
• Zhao J, Song Y, Liu D. Gilteritinib: a novel FLT3 inhibitor for acute myeloid leukemia. Biomark Res. 2019;7:19 The authors summarized clinical trials of gilteritinib for AML espicially the data from the ADMIRAL clinical study, open-label, multicenter, randomized phase III study, which has shown the importance of gilteritinib as a new standard therapy for R/R FLT3-mutated AML.
Molenaar RJ, Maciejewski JP, Wilmink JW, van Noorden CJF. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene. 2018;37(15):1949–60.
Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019;133(7):676–87.
Richard-Carpentier G, DiNardo CD. Venetoclax for the treatment of newly diagnosed acute myeloid leukemia in patients who are ineligible for intensive chemotherapy. Ther Adv Hematol. 2019;10:2040620719882822.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
Wei AH, Strickland SA Jr, Hou JZ, Fiedler W, Lin TL, Walter RB, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–84.
Ok CY, Singh RR, Vega F. Aberrant activation of the hedgehog signaling pathway in malignant hematological neoplasms. Am J Pathol. 2012;180(1):2–11.
Irvine DA, Copland M. Targeting hedgehog in hematologic malignancy. Blood. 2012;119(10):2196–204.
Aberger F, Ruiz IAA. Context-dependent signal integration by the GLI code: the oncogenic load, pathways, modifiers and implications for cancer therapy. Semin Cell Dev Biol. 2014;33:93–104.
Queiroz KC, Ruela-de-Sousa RR, Fuhler GM, Aberson HL, Ferreira CV, Peppelenbosch MP, et al. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene. 2010;29(48):6314–22.
Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–89.
Zhang J, Gu Y, Chen B. Mechanisms of drug resistance in acute myeloid leukemia. Onco Targets Ther. 2019;12:1937–45.
Teixido J, Hemler ME, Greenberger JS, Anklesaria P. Role of beta 1 and beta 2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. J Clin Invest. 1992;90(2):358–67.
Pievani A, Biondi M, Tomasoni C, Biondi A, Serafini M. Location first: targeting acute myeloid leukemia within its niche. J Clin Med. 2020;9(5).
Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12(10):1167–74.
Ellis SL, Grassinger J, Jones A, Borg J, Camenisch T, Haylock D, et al. The relationship between bone, hemopoietic stem cells, and vasculature. Blood. 2011;118(6):1516–24.
Gutjahr JC, Bayer E, Yu X, Laufer JM, Hopner JP, Tesanovic S, et al. CD44 engagement enhances acute myeloid leukemia cell adhesion to the bone marrow microenvironment by increasing VLA-4 avidity. Haematologica. 2020.
Battula VL, Le PM, Sun JC, Nguyen K, Yuan B, Zhou X, et al. AML-induced osteogenic differentiation in mesenchymal stromal cells supports leukemia growth. JCI Insight. 2017;2(13).
Le PM, Andreeff M, Battula VL. Osteogenic niche in the regulation of normal hematopoiesis and leukemogenesis. Haematologica. 2018;103(12):1945–55.
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41.
DeAngelo D. Uproleselan (GMI-1271), an E-Selectin antagonist, improves the efficacy and safety of chemotherapy in relapsed/refractory (R/R) and newly diagnosed older patients with acute myeloid leukemia: final, correlative, and subgroup analyses. Blood. 2018;132(331).
Cao T, Ye Y, Liao H, Shuai X, Jin Y, Su J, et al. Relationship between CXC chemokine receptor 4 expression and prognostic significance in acute myeloid leukemia. Medicine (Baltimore). 2019;98(23):e15948.
Ladikou EE, Sivaloganathan H, Pepper A, Chevassut T. Acute myeloid leukaemia in its niche: the bone marrow microenvironment in acute myeloid leukaemia. Curr Oncol Rep. 2020;22(3):27.
Martinez-Cuadron D, Boluda B, Martinez P, Bergua J, Rodriguez-Veiga R, Esteve J, et al. A phase I-II study of plerixafor in combination with fludarabine, idarubicin, cytarabine, and G-CSF (PLERIFLAG regimen) for the treatment of patients with the first early-relapsed or refractory acute myeloid leukemia. Ann Hematol. 2018;97(5):763–72.
Giulia Borella ADR, Porcù E, Tregnago C, Benetton M, Bisio V, Campodoni E, et al. Acute myeloid leukemia (AML) in a 3D bone marrow niche showed high performance for in vitro and in vivo drug screenings. Blood. 2019;134:544.
Benito J, Ramirez MS, Millward NZ, Velez J, Harutyunyan KG, Lu H, et al. Hypoxia-activated prodrug TH-302 targets hypoxic bone marrow niches in preclinical leukemia models. Clin Cancer Res. 2016;22(7):1687–98.
Portwood S, Lal D, Hsu YC, Vargas R, Johnson MK, Wetzler M, et al. Activity of the hypoxia-activated prodrug, TH-302, in preclinical human acute myeloid leukemia models. Clin Cancer Res. 2013;19(23):6506–19.
Badar T, Handisides DR, Benito JM, Richie MA, Borthakur G, Jabbour E, et al. Phase I study of evofosfamide, an investigational hypoxia-activated prodrug, in patients with advanced leukemia. Am J Hematol. 2016;91(8):800–5.
McMahon CM, Ferng T, Canaani J, Wang ES, Morrissette JJD, Eastburn DJ, et al. Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov. 2019;9(8):1050–63.
Reilly EO, Dhami SPS, Baev DV, Ortutay C, Halpin-Mccormick A, Morrell R, et al. Repression of Mcl-1 expression by the CDC7/CDK9 inhibitor PHA-767491 overcomes bone marrow stroma-mediated drug resistance in AML. Sci Rep. 2018;8(1):15752.
Han L, Zhang Q, Dail M, Shi C, Cavazos A, Ruvolo VR, et al. Concomitant targeting of BCL2 with venetoclax and MAPK signaling with cobimetinib in acute myeloid leukemia models. Haematologica. 2020;105(3):697–707.
Jamieson C. Hedgehog pathway inhibitors: a new therapeutic class for the treatment of acute myeloid leukemia Blood Cancer Discovery. 2020;1(2).
Zhao X PT, Ornell KJ, Zhou P, Dabral SK, Pak E, et al. RAS/MAPK activation drives resistance to Smo inhibition, metastasis, and tumor evolution in Shh pathway-dependent tumors. Cancer Research. 2015;75(17).
Pricl S, Cortelazzi B, Dal Col V, Marson D, Laurini E, Fermeglia M, et al. Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma. Mol Oncol. 2015;9(2):389–97.
Tan SF, Liu X, Fox TE, Barth BM, Sharma A, Turner SD, et al. Acid ceramidase is upregulated in AML and represents a novel therapeutic target. Oncotarget. 2016;7(50):83208–22.
Mahadevan D, List AF. Targeting the multidrug resistance-1 transporter in AML: molecular regulation and therapeutic strategies. Blood. 2004;104(7):1940–51.
Tan SF, Dunton W, Liu X, Fox TE, Morad SAF, Desai D, et al. Acid ceramidase promotes drug resistance in acute myeloid leukemia through NF-kappaB-dependent P-glycoprotein upregulation. J Lipid Res. 2019;60(6):1078–86.
Vannini A, Leoni V, Barboni C, Sanapo M, Zaghini A, Malatesta P, et al. alphavbeta3-integrin regulates PD-L1 expression and is involved in cancer immune evasion. Proc Natl Acad Sci U S A. 2019;116(40):20141–50.
Davis PJ, Mousa SA, Lin HY. Tetraiodothyroacetic acid (tetrac), integrin alphavbeta3 and disabling of immune checkpoint defense. Future Med Chem. 2018;10(14):1637–9.
Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12(2):111–21.
Johansen S, Brenner AK, Bartaula-Brevik S, Reikvam H, Bruserud O. The possible importance of beta3 integrins for leukemogenesis and chemoresistance in acute myeloid leukemia. Int J Mol Sci. 2018;19(1).
Azzariti A, Mancarella S, Porcelli L, Quatrale AE, Caligiuri A, Lupo L, et al. Hepatic stellate cells induce hepatocellular carcinoma cell resistance to sorafenib through the laminin-332/alpha3 integrin axis recovery of focal adhesion kinase ubiquitination. Hepatology. 2016;64(6):2103–17.
Schmohl KA, Nelson PJ, Spitzweg C. Tetrac as an anti-angiogenic agent in cancer. Endocr Relat Cancer. 2019;26(6):R287–304.
Yalcin M, Bharali DJ, Lansing L, Dyskin E, Mousa SS, Hercbergs A, et al. Tetraidothyroacetic acid (tetrac) and tetrac nanoparticles inhibit growth of human renal cell carcinoma xenografts. Anticancer Res. 2009;29(10):3825–31.
Zhao Z, Chen Y, Francisco NM, Zhang Y, Wu M. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B. 2018;8(4):539–51.
Mardiana S, Gill S. CAR T cells for acute myeloid leukemia: state of the art and future directions. Front Oncol. 2020;10:697.
•• Zhang H, Gan WT, Hao WG, Wang PF, Li ZY, Chang LJ. Successful anti-CLL1 CAR T cell therapy in secondary acute myeloid leukemia. Front Oncol. 2020;10:685 In this case report, treating AML patient with anti-CLL1 CAR-T therapy achieved complete morphological, immunophenotypic, and molecular remission for over 10 months. This CAR T cells models might be another effective treatment option for AML in the future.
Fang Liu YC, Kevin Pinz, Yu Ma, Masayuki Wada, Kevin Chen, Gina Ma, Jiaqi Shen, Charlotte Olivia Tse, Yi Su, Yisong Xiong, Guangcui He, Yecheng Li, Yupo Ma. First-in-human CLL1-CD33 compound CAR T cell therapy induces complete remission in patients with refractory acute myeloid leukemia. Blood. 2018;132(901).
Fang Liu HZ, Sun L, Li Y, Zhang S, He G, Yi H, Wada M, Pinz KG, Chen KH, Ma Y, Xiong Y, Su Y, Ma Y. First-in-human CLL1-CD33 compound car (CCAR) T cell therapy in relapsed and refractory acute myeloid leukemia EHA Library2020 [cited 2020 09/24]. Available from: https://library.ehaweb.org/eha/2020/eha25th/294969/fang.liu.first-in-human.cll1-cd33.compound.car.28ccar29.t.cell.therapy.in.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Ds149.
Budde L, Song JY, Kim Y, Blanchard S, Wagner J, Stein AS, et al. Remissions of acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasm following treatment with CD123-specific CAR T cells: a first-in-human clinical trial. Blood. 2017;130(811).
Sommer C. Allogeneic FLT3 CAR T Cells with an off-switch exhibit potent activity against AML and can be depleted to expedite bone marrow recovery. 2020.
Daver N. A bispecific approach to improving CAR T cells in AML. Blood. 2020;135(10):703–4.
He X, Feng Z, Ma J, Ling S, Cao Y, Gurung B, et al. Bispecific and split CAR T cells targeting CD13 and TIM3 eradicate acute myeloid leukemia. Blood. 2020;135(10):713–23.
Liao D, Wang M, Liao Y, Li J, Niu T. A review of efficacy and safety of checkpoint inhibitor for the treatment of acute myeloid leukemia. Front Pharmacol. 2019;10:609.
Justin Kline M, Hongtao Liu MD. PhD, Tallarico Michael, MD, Andrew S Artz, MD, James Godfrey, MD, Emily K Curran, MD, Wendy Stock, MD, Sonali M. Smith, MD, Michael R. Bishop, MD. Pembrolizumab for the treatment of disease relapse following allogeneic hematopoietic cell transplantation. Blood. 2018;132.
Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–51.
Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label, phase II study. Cancer Discov. 2019;9(3):370–83.
Chen KTJ, Gilabert-Oriol R, Bally MB, Leung AWY. Recent treatment advances and the role of nanotechnology, combination products, and immunotherapy in changing the therapeutic landscape of acute myeloid leukemia. Pharm Res. 2019;36(9):125.
Mayer LD, Tardi P, Louie AC. CPX-351: a nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int J Nanomedicine. 2019;14:3819–30.
•• Darwish NHE, Sudha T, Godugu K, Bharali DJ, Elbaz O, El-Ghaffar HAA, et al. Novel targeted nano-parthenolide molecule against NF-kB in acute myeloid leukemia. Molecules. 2019;24(11). The nano-targeting techenology offers an excellent delivery system with high selectivity. This is an example of multiple targeting which can be achieved by this technology which might be effective in the future against different type of cancers.
Sudha T, Bharali DJ, Yalcin M, Darwish NH, Debreli Coskun M, Keating KA, et al. Targeted delivery of paclitaxel and doxorubicin to cancer xenografts via the nanoparticle of nano-diamino-tetrac. Int J Nanomedicine. 2017;12:1305–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rehan Uddin declares that he has no conflict of interest. Noureldian H. E. Darwish declares that he has no conflict of interest. Shaker A. Mousa declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Uddin, R., Darwish, N.H.E. & Mousa, S.A. Acute Myeloid Leukemia Mutations and Future Mechanistic Target to Overcome Resistance. Curr. Treat. Options in Oncol. 22, 76 (2021). https://doi.org/10.1007/s11864-021-00880-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00880-x