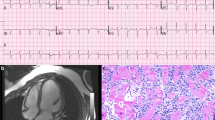
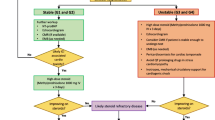
Opinion statement
Immunotherapies have transformed the current landscape for cancer treatment and demonstrated unparalleled improvements in survival rates. Now, a third of cancer patients are eligible for treatment with the most widely used class of immunotherapy, immune checkpoint inhibitors (ICIs). As more patients are treated with these novel agents, it is critical for both oncologists and subspecialists to establish a better understanding of the adverse events which can occur. The incidence of myocarditis associated with ICI therapy has been reported to be between 0.27 and 1.14%, 5 times that of myocarditis from other cancer therapies, and, of those patients, 20–50% develop a fulminant form. However, because of unclear risk factors, a broad clinical spectrum, and lack of specific noninvasive studies for diagnosis, the care of patients with ICI-associated cardiotoxicity can be challenging. Here, we have provided a brief overview of the current immunotherapy agents with a focus on the emerging evidence regarding diagnosis and management of cardiac adverse events.




Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Weir HK, Thompson TD, Soman A, Møller B, Leadbetter S. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer. 2015;121(11):1827–37. https://doi.org/10.1002/cncr.29258.
Society AC. Cancer Facts & Figs. 2020. Accessed on September 9, 2020.
Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17(9):2745–51. https://doi.org/10.1200/JCO.1999.17.9.2745.
• Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–46. https://doi.org/10.1056/NEJMoa1910836 This article shows the sustained long-term overall survival at 5 years in melanoma patients treated with immune checkpoint blockade: immunotherapy has radically changed the options for cancer treatment. Melanoma was the first tumor where these striking response rates with immunotherapy were observed, leading to a new era in oncology.
Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. https://doi.org/10.1016/j.intimp.2018.06.001.
Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open. 2020;3(3):e200423. https://doi.org/10.1001/jamanetworkopen.2020.0423.
Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. https://doi.org/10.1001/jamanetworkopen.2019.2535.
Yu J, Hodge J, Oliva C, Neftelinov S, Hubbard-Lucey, V, Tang J. Trends in clinical development for PD-1/PD-L1 inhibitors. Nature Reviews Drug Discovery. 2019. https://www.nature.com/articles/d41573-019-00182-w
• Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–8. https://doi.org/10.1001/jamaoncol.2018.3923 This article describes the fulminant and fatal toxic effects that can happen after treatment with immune checkpoint inhibitors. It is the largest review that has been published on this topic. It highlights the need for clinicians to be aware of these serious complications the need for early detection/accurate management.
Shalabi H, Angiolillo A, Fry TJ. Beyond CD19: opportunities for future development of targeted immunotherapy in pediatric relapsed-refractory acute leukemia. Front Pediatr. 2015;3:80. https://doi.org/10.3389/fped.2015.00080.
Maude SL, Shpall EJ, Grupp SA. Chimeric antigen receptor T cell therapy for ALL. Hematology Am Soc Hematol Educ Program. 2014;2014(1):559–64. https://doi.org/10.1182/asheducation-2014.1.559.
Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B cell lymphoma and indolent B cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–9. https://doi.org/10.1200/JCO.2014.56.2025.
Schultz L, Mackall C. Driving CAR T cell translation forward. Sci Transl Med. 2019;11(481). https://doi.org/10.1126/scitranslmed.aaw2127.
Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–86. https://doi.org/10.1158/2159-8290.CD-18-0367.
Bayer V, Amaya B, Baniewicz D, Callahan C, Marsh L, McCoy AS. Cancer immunotherapy: an evidence-based overview and implications for practice. Clin J Oncol Nurs. 2017;21(2 Suppl):13–21. https://doi.org/10.1188/17.CJON.S2.13-21.
Gaynor N, Crown J, Collins DM. Immune checkpoint inhibitors: Key trials and an emerging role in breast cancer. Semin Cancer Biol. 2020. https://doi.org/10.1016/j.semcancer.2020.06.016.
Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50. https://doi.org/10.1186/s40425-016-0152-y.
Chen DY, Huang WK, Chien-Chia Wu V, et al. Cardiovascular toxicity of immune checkpoint inhibitors in cancer patients: a review when cardiology meets immuno-oncology. J Formos Med Assoc. 2020;119(10):1461–75. https://doi.org/10.1016/j.jfma.2019.07.025.
• Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol. 2019;74(25):3099–108. https://doi.org/10.1016/j.jacc.2019.10.038 This work reviews cardiac toxicity in patients treated with chimeric antigen receptors redirect T cells (CAR-T) therapy and shows a graded relationship among CRS (cytokine release syndrome), elevated troponin, and cardiovascular events, with toxicity rates being lower when tocilizumab is given early after CRS onset.
Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–38. https://doi.org/10.1016/j.bbmt.2018.12.758.
Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–95. https://doi.org/10.1182/blood-2014-05-552,729.
Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. https://doi.org/10.1186/s40425-018-0343-9.
Riegler LL, Jones GP, Lee DW. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T cell therapy. Ther Clin Risk Manag. 2019;15:323–35. https://doi.org/10.2147/TCRM.S150524.
Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5(6):e1163462. https://doi.org/10.1080/2162402X.2016.1163462.
Berraondo P, Sanmamed MF, Ochoa MC, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120(1):6–15. https://doi.org/10.1038/s41416-018-0328-y.
Dutcher JP, Schwartzentruber DJ, Kaufman HL, et al. High dose interleukin-2 (aldesleukin) - expert consensus on best management practices-2014. J Immunother Cancer. 2014;2(1):26. https://doi.org/10.1186/s40425-014-0026-0.
Huehls AM, Coupet TA, Sentman CL. Bispecific T cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93(3):290–6. https://doi.org/10.1038/icb.2014.93.
Goebeler ME, Bargou RC. T cell-engaging therapies - BiTEs and beyond. Nat Rev Clin Oncol. 2020;17(7):418–34. https://doi.org/10.1038/s41571-020-0347-5.
Sedykh SE, Prinz VV, Buneva VN, Nevinsky GA. Bispecific antibodies: design, therapy, perspectives. Drug Des Devel Ther. 2018;12:195–208. https://doi.org/10.2147/DDDT.S151282.
Upadhrasta S, Elias H, Patel K, Zheng L. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis Transl Med. 2019;5(1):6–14. https://doi.org/10.1016/j.cdtm.2019.02.004.
de Almeida DVP, Gomes JR, Haddad FJ, Buzaid AC. Immune-mediated pericarditis with pericardial tamponade during nivolumab therapy. J Immunother. 2018;41(7):329–31. https://doi.org/10.1097/CJI.0000000000000217.
Yun S, Vincelette ND, Mansour I, Hariri D, Motamed S. Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med. 2015;2015:794842. https://doi.org/10.1155/2015/794842.
Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921–64. https://doi.org/10.1093/eurheartj/ehv318.
Chiabrando JG, Bonaventura A, Vecchié A, et al. Management of acute and recurrent pericarditis: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(1):76–92. https://doi.org/10.1016/j.jacc.2019.11.021.
Hu JR, Florido R, Lipson EJ, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115(5):854–68. https://doi.org/10.1093/cvr/cvz026.
Brahmer JR, Lacchetti C, Thompson JA, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract. 2018;14(4):247–9. https://doi.org/10.1200/JCO.2017.77.6385.
Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv264–6. https://doi.org/10.1093/annonc/mdy162.
Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. https://doi.org/10.1186/s40425-017-0300-z.
Imazio M, Gaita F, LeWinter M. Evaluation and treatment of pericarditis: a systematic review. JAMA. 2015;314(14):1498–506. https://doi.org/10.1001/jama.2015.12763.
Altan M, Toki MI, Gettinger SN, et al. Immune checkpoint inhibitor-associated pericarditis. J Thorac Oncol. 2019;14(6):1102–8. https://doi.org/10.1016/j.jtho.2019.02.026.
Reinhold-Keller E, Herlyn K, Wagner-Bastmeyer R, Gross WL. Stable incidence of primary systemic vasculitides over five years: results from the German vasculitis register. Arthritis Rheum. 2005;53(1):93–9. https://doi.org/10.1002/art.20928.
Sharma P, Sharma S, Baltaro R, Hurley J. Systemic vasculitis. Am Fam Physician. 2011;83(5):556–65.
Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol. 2018. https://doi.org/10.1007/s10067-018-4177-0.
Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA. 2016;315(22):2442–58. https://doi.org/10.1001/jama.2016.5444.
Weyand CM, Goronzy JJ. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med. 2014;371(1):50–7. https://doi.org/10.1056/NEJMcp1214825.
Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–89. https://doi.org/10.1016/S1470-2045(18)30608-9.
Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a position statement from the Taskforce on Takotsubo syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18(1):8–27. https://doi.org/10.1002/ejhf.424.
Coen M, Rigamonti F, Roth A, Koessler T. Chemotherapy-induced Takotsubo cardiomyopathy, a case report and review of the literature. BMC Cancer. 2017;17(1):394. https://doi.org/10.1186/s12885-017-3384-4.
Escudier M, Cautela J, Malissen N, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136(21):2085–7. https://doi.org/10.1161/CIRCULATIONAHA.117.030571.
Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–58. https://doi.org/10.1016/S1470-2045(18)30457-1.
Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–27. https://doi.org/10.1161/CIRCRESAHA.118.313591.
Drobni ZD, Alvi RM, Taron J, et al. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation. 2020. https://doi.org/10.1161/CIRCULATIONAHA.120.049981.
Poels K, van Leent MMT, Boutros C, et al. Immune checkpoint inhibitor therapy aggravates T cell–driven plaque inflammation in atherosclerosis. JACC: CardioOncology. 2020;2(4):599–610. https://doi.org/10.1016/j.jaccao.2020.08.007.
• Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–64. https://doi.org/10.1016/j.jacc.2018.02.037 This manuscript describes the key clinical, imaging, and pathological features of myocarditis secondary to immune checkpoint inhibitors. In addition, it highlights the high major cardiac adverse event rate of this toxicity if it is not promptly detected and managed correctly.
Läubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015;3:11. https://doi.org/10.1186/s40425-015-0057-1.
Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on etiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–48, 2648a-2648d. https://doi.org/10.1093/eurheartj/eht210.
Tadokoro T, Keshino E, Makiyama A, et al. Acute Lymphocytic Myocarditis With Anti-PD-1 Antibody Nivolumab. Circ Heart Fail. 2016;9(10). https://doi.org/10.1161/CIRCHEARTFAILURE.116.003514.
Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9(2):e013757. https://doi.org/10.1161/JAHA.119.013757.
Ganatra S, Neilan TG. Immune checkpoint inhibitor-associated myocarditis. Oncologist. 2018;23(8):879–86. https://doi.org/10.1634/theoncologist.2018-0130.
Grabie N, Gotsman I, DaCosta R, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T cell mediated injury in the heart. Circulation. 2007;116(18):2062–71. https://doi.org/10.1161/CIRCULATIONAHA.107.709360.
Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188(10):4876–84. https://doi.org/10.4049/jimmunol.1200389.
Okazaki T, Tanaka Y, Nishio R, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9(12):1477–83. https://doi.org/10.1038/nm955.
Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination Immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–55. https://doi.org/10.1056/NEJMoa1609214.
Müller OJ, Spehlmann ME, Frey N. Cardio-toxicity of checkpoint inhibitors. J Thorac Dis. 2018;10(Suppl 35):S4400–4. https://doi.org/10.21037/jtd.2018.12.78.
Al-Kindi SG, Oliveira GH. Reporting of immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;392(10145):382–3. https://doi.org/10.1016/S0140-6736(18)31542-3.
• Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, Version 1.2019. J Natl Compr Canc Netw. 2019;17(3):255–89. https://doi.org/10.6004/jnccn.2019.0013 These guidelines represent a valuable resource for clinicians. They are the most up-to-date and describe the main diagnostic and management recommendations for the most common toxicities related to ICI.
Michel L, Rassaf T. Cardio-oncology: need for novel structures. Eur J Med Res. 2019;24(1):1. https://doi.org/10.1186/s40001-018-0359-0.
Koelzer VH, Rothschild SI, Zihler D, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer. 2016;4:13. https://doi.org/10.1186/s40425-016-0117-1.
Rota E, Varese P, Agosti S, et al. Concomitant myasthenia gravis, myositis, myocarditis and polyneuropathy, induced by immune-checkpoint inhibitors: a life-threatening continuum of neuromuscular and cardiac toxicity. eNeurologicalSci. 2019;14:4–5. https://doi.org/10.1016/j.ensci.2018.11.023.
Oren O, Yang EH, Molina JR, Bailey KR, Blumenthal RS, Kopecky SL. Cardiovascular health and outcomes in cancer patients receiving immune checkpoint inhibitors. Am J Cardiol. 2020;125(12):1920–6. https://doi.org/10.1016/j.amjcard.2020.02.016.
Ball S, Ghosh RK, Wongsaengsak S, et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol. 2019;74(13):1714–27. https://doi.org/10.1016/j.jacc.2019.07.079.
Bonaca MP, Olenchock BA, Salem JE, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140(2):80–91. https://doi.org/10.1161/CIRCULATIONAHA.118.034497.
Berliner D, Beutel G, Bauersachs J. Echocardiography and biomarkers for the diagnosis of cardiotoxicity. Herz. 2020. https://doi.org/10.1007/s00059-020-04957-5.
Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5(5):596–603. https://doi.org/10.1161/CIRCIMAGING.112.973321.
Lee Chuy K, Oikonomou EK, Postow MA, et al. Myocarditis surveillance in patients with advanced melanoma on combination immune checkpoint inhibitor therapy: the Memorial Sloan Kettering Cancer Center Experience. Oncologist. 2019;24(5):e196–7. https://doi.org/10.1634/theoncologist.2019-0040.
Michel L, Mincu RI, Mahabadi AA, et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22(2):350–61. https://doi.org/10.1002/ejhf.1631.
Zlotoff D. Association between EKG Alterations and Clinical Outcomes in Immune Checkpoint Inhibitor-Associated Myocarditis. Journal of Cardiac Failure. 2019;25(8):S72–3.
Awadalla M, Mahmood SS, Groarke JD, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. 2020;75(5):467–78. https://doi.org/10.1016/j.jacc.2019.11.049.
Zhang L, Awadalla M, Mahmood SS, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41(18):1733–43. https://doi.org/10.1093/eurheartj/ehaa051.
Champion SN, Stone JR. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod Pathol. 2020;33(1):99–108. https://doi.org/10.1038/s41379-019-0363-0.
Sobol I, Chen CL, Mahmood SS, Borczuk AC. Histopathologic Characterization of Myocarditis Associated With Immune Checkpoint Inhibitor Therapy. Arch Pathol Lab Med. 2020. https://doi.org/10.5858/arpa.2019-0447-OA.
Carlino MS, Long GV. Ipilimumab combined with nivolumab: a standard of care for the treatment of advanced melanoma? Clin Cancer Res. 2016;22(16):3992–8. https://doi.org/10.1158/1078-0432.CCR-15-2944.
Fukasawa Y, Sasaki K, Natsume M, et al. Nivolumab-Induced myocarditis concomitant with myasthenia gravis. Case Rep Oncol. 2017;10(3):809–12. https://doi.org/10.1159/000479958.
Zhang L, Zlotoff DA, Awadalla M, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation. 2020;141(24):2031–4. https://doi.org/10.1161/CIRCULATIONAHA.119.044703.
Esfahani K, Buhlaiga N, Thébault P, Lapointe R, Johnson NA, Miller WH. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380(24):2375–6. https://doi.org/10.1056/NEJMc1903064.
Salem JE, Allenbach Y, Vozy A, et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380(24):2377–9. https://doi.org/10.1056/NEJMc1901677.
Behnam SM, Behnam SE, Koo JY. TNF-alpha inhibitors and congestive heart failure. Skinmed. 2005;4(6):363–8. https://doi.org/10.1111/j.1540-9740.2005.04502.x.
Noji Y. Anakinra in fulminant myocarditis: targeting interleukin-1 and the inflammasome formation. Crit Care Med. 2016;44(8):1630–1. https://doi.org/10.1097/CCM.0000000000001769.
NIH U.S. National Library of Medicine ClinicalTrials.gov. Accessed December 10, 2020.
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–801. https://doi.org/10.1093/eurheartj/ehw211.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Marina Frayberg declares that she has no conflict of interest. Anthony Yung declares that he has no conflict of interest. Leyre Zubiri has received compensation from Merck for service as a consultant. Daniel A. Zlotoff declares that he has no conflict of interest. Kerry L. Reynolds declares that she has no conflict of interest.
Disclosures
Marina Frayberg: No relevant disclosures.
Anthony Yung: No relevant disclosures.
Leyre Zubiri: MERCK consultant.
Daniel A Zlotoff: No relevant disclosures.
Kerry L Reynolds: No relevant disclosures.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Marina Frayberg and Anthony Yung contributed equally as authors.
Rights and permissions
About this article
Cite this article
Frayberg, M., Yung, A., Zubiri, L. et al. What the Cardiologist Needs to Know About Cancer Immunotherapies and Complications. Curr. Treat. Options in Oncol. 22, 53 (2021). https://doi.org/10.1007/s11864-021-00844-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00844-1