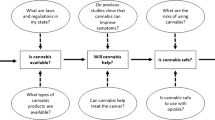
Opinion statement
The use of medical cannabis is expanding in the USA. Due to conflicting, low-quality evidence, many oncologists may not feel confident to recommend it to patients. Given the potential for legal and financial risks when conducting clinical trials with medical cannabis, the use of observational data should be explored. Observational data that directly capture medical cannabis use in relation to prescription medications and track the prevalence and patterns of cannabis use is sparse. To gain insights into the role medical cannabis plays in the pharmaceutical landscape, proxies such as cannabis legislation need to be explored. In the context of recommendation-nonadherent antiemetic prescribing among patients experiencing chemotherapy-induced nausea and vomiting, medical cannabis may be a suitable alternative to an antiemetic in states that allow medical cannabis. Findings suggest that legislation may impact the use of certain antiemetics in states with cannabis legislation in place. The presence or absence of legislation regarding medical cannabis use may serve as an early, observable surrogate marker of medical cannabis use in the community. In light of the paucity of clinical trials and observational datasets that capture cannabis use, there remains a tremendous need for the development of methodologies or standardized datasets that appropriately and reliably capture the use of medical cannabis to facilitate research into its clinical application and effect on prescription medication use. Standardizing the reporting and destigmatizing use could eliminate the dependence upon proxy measures as a substitute for more extensive data and go a long way in improving data capture, thus allowing us to generate knowledge and hypotheses from observational data until research conditions improve and allow for expanded clinical trials involving medical cannabis.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hill KP. Medical use of cannabis in 2019. JAMA. 2019;322(10):974–5. https://doi.org/10.1001/jama.2019.11868.
Compton WM, Han B, Hughes A, Jones CM, Blanco C. Use of marijuana for medical purposes among adults in the United States. JAMA. 2017;317(2):209–11. https://doi.org/10.1001/jama.2016.18900.
Abrams DI. Should oncologists recommend cannabis? Curr Treat Options Oncol. 2019;20(7):59. https://doi.org/10.1007/s11864-019-0659-9.
Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2007;15(5):497–503. https://doi.org/10.1007/s00520-006-0173-z.
Gralla RJ, de Wit R, Herrstedt J, et al. Antiemetic efficacy of the neurokinin-1 antagonist, aprepitant, plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high-dose cisplatin: analysis of combined data from two Phase III randomiz. Cancer. 2005;104(4):864–8. https://doi.org/10.1002/cncr.21222.
Check DK, Basch EM. Appropriate use of antiemetics to prevent chemotherapy-induced nausea and vomiting. JAMA Oncol. 2017;3(3):307–9. https://doi.org/10.1001/jamaoncol.2016.2616.
Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374(14):1356–67. https://doi.org/10.1056/NEJMra1515442.
Badowski ME. A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics. Cancer Chemother Pharmacol. 2017;80(3):441–9. https://doi.org/10.1007/s00280-017-3387-5.
Kramer JL. Medical marijuana for cancer. CA Cancer J Clin. 2015;65(2):109–22. https://doi.org/10.3322/caac.21260.
Holle LM, Boehnke ML. Oncology pharmacists in health care delivery: vital members of the cancer care team. J Oncol Pract. 2014;10(3):e142–5. https://doi.org/10.1200/JOP.2013.001257.
Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–73. https://doi.org/10.1001/jama.2015.6358.
Chow R, Valdez C, Chow N, et al. Oral cannabinoid for the prophylaxis of chemotherapy-induced nausea and vomiting-a systematic review and meta-analysis. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2020;28(5):2095–103. https://doi.org/10.1007/s00520-019-05280-4. Systematic review and meta-analysis of randomized clinical trials that assess the efficacy and safety of oral cannabinoids in chemotherapy induced nausea and vomiting.
Amar M. Ben. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105:1–25.
Braun IM, Wright A, Peteet J, et al. Medical oncologists’ beliefs, practices, and knowledge regarding marijuana used therapeutically: a nationally representative survey study. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(19):1957–62. https://doi.org/10.1200/JCO.2017.76.1221. Nationally representative survey of oncologists summarizing their beliefs, practice, and knowledge of medical marijuana.
Zylla D, Steele G, Eklund J, Mettner J, Arneson T. Oncology clinicians and the minnesota medical cannabis program: a survey on medical cannabis practice patterns, barriers to enrollment, and educational needs. Cannabis Cannabinoid Res. 2018;3(1):195–202. https://doi.org/10.1089/can.2018.0029.
Moeller KE, McGuire JM, Melton BL. A nationwide survey of pharmacy students’ knowledge and perceptions regarding medical cannabis. J Am Pharm Assoc (2003). 2020;60(1):218–224.e3. https://doi.org/10.1016/j.japh.2019.08.008.
Gladden ME, Hung D, Bhandari NR, et al. Arkansas community’s attitudes toward the regulation of medical cannabis and the pharmacist’s involvement in Arkansas medical cannabis. J Am Pharm Assoc (2003). 2020;60(1):235–43. https://doi.org/10.1016/j.japh.2019.11.005.
United States Drug Enforcement Agency. Drug scheduling. https://www.dea.gov/drug-scheduling. Accessed June 30, 2020.
Shen H. Federal red tape ties up marijuana research. Nature. 2014;507(7493):407–8. https://doi.org/10.1038/507407a.
Stith SS, Vigil JM. Federal barriers to Cannabis research. Science. 2016;352(6290):1182. https://doi.org/10.1126/science.aaf7450.
Food and Drug Administration. FDA and Cannabis: Research and Drug Approval Process. https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process. Published 2020. Accessed June 29, 2020.
Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638–54. https://doi.org/10.1016/j.sapharm.2015.09.002.
Costantino RC, Felten N, Todd M, Maxwell T, McPherson ML, et al. J Palliat Med. 2019;22(10):1208–12. https://doi.org/10.1089/jpm.2018.0535.
National Conference of State Legislature. State Medical Marijuana Laws. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Published 2018. Accessed July 15, 2019.
Bestrashniy J, Winters KC. Variability in medical marijuana laws in the United States. Psychol Addict Behav. 2015;29(3):639–42. https://doi.org/10.1037/adb0000111.
Bradford AC, Bradford WD. Medical marijuana laws reduce prescription medication use in Medicare Part D. Health Aff (Millwood). 2016;35(7):1230–6. https://doi.org/10.1377/hlthaff.2015.1661 Observational research study demonstrating the effect of medical marijuana laws on prescription medication use.
Stith SS, Vigil JM, Adams IM, Reeve AP. Effects of legal access to cannabis on Scheduled II-V drug prescriptions. J Am Med Dir Assoc. 2018;19(1):59–64.e1. https://doi.org/10.1016/j.jamda.2017.07.017.
Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J. Impact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a U.S. population. Support Care Cancer. 2011;19(6):843–51. https://doi.org/10.1007/s00520-010-0915-9.
American Cancer Society. Economic impact of cancer. https://www.cancer.org/cancer/cancer-basics/economic-impact-of-cancer.html. Published 2018. Accessed July 15, 2019.
Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33(23):2563–77. https://doi.org/10.1200/JCO.2015.61.6706.
American Society of Clinical Oncology (ASCO). Choosing wisely: ten things physicans and patients should question. http://www.choosingwisely.org/societies/american-society-of-clinical-oncology/. Published 2013.
Broder MS, Faria C, Powers A, Sunderji J, Cherepanov D. The impact of 5-HT3RA use on cost and utilization in patients with chemotherapy-induced nausea and vomiting: systematic review of the literature. Am Heal drug benefits. 2014;7(3):171–82.
Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29(31):4189–98. https://doi.org/10.1200/JCO.2010.34.4614.
Kinzbrunner BM. Review: cannabinoids and codeine have similar effects on pain relief, but cannabinoids commonly cause psychotropic adverse effects. Evid Based Med. 2002;7(1):24. https://doi.org/10.1136/ebm.7.1.24.
Mersiades AJ, Stockler MR, Olver IN, Grimison P. Medicinal cannabis for chemotherapy-induced nausea and vomiting: prescribing with limited evidence. Med J Aust. 2019;210(1):11–12.e1. https://doi.org/10.5694/mja17.01099.
Acknowledgments
The authors would like to acknowledge Dr. Volker Gressler for his clinical expertise and assistance throughout the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimers
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any agency of the US government.
Conflict of interest
Laura Gressler, Alan Baltz, and Ryan Costantino have no conflicts of interest to declare. Dr. Onukwugha reports grants from Bayer Healthcare, grants from Pfizer, Inc., and personal fees from IMPAQ, International, outside the submitted work. Dr. Slejko reports grants from PhRMA, grants from PhRMA Foundation, grants from Novartis Pharmaceuticals, grants from Takeda Pharmaceuticals, and other from Pfizer, outside the submitted work. Alan P. Baltz declares that he has no conflict of interest. Ryan C. Costantino declares that he has no conflict of interest. Julia F. Slejko has received research funding from PhRMA, PhRMA Foundation, Novartis Pharmaceuticals, and Takeda Pharmaceuticals and has received a teaching honorarium from Pfizer. Eberechukwu Onukwugha has received research funding from Bayer Healthcare Pharmaceuticals and Pfizer and has received compensation from Novo Nordisk for service as a consultant.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Palliative and Supportive Care
Rights and permissions
About this article
Cite this article
Gressler, L.E., Baltz, A.P., Costantino, R.C. et al. Exploring the Use of State Medical Cannabis Legislation as a Proxy for Medical Cannabis Use Among Patients Receiving Chemotherapy. Curr. Treat. Options in Oncol. 22, 1 (2021). https://doi.org/10.1007/s11864-020-00803-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-020-00803-2