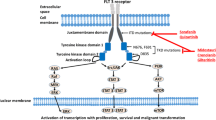
Opinion statement
Acute myeloid leukemia (AML) disease prognosis is poor and there is a high risk of chemo-resistant relapse for both young and old patients. Thus, there is a demand for alternative and target-specific drugs to improve the 5-year survival rate. Current treatment mainstays include chemotherapy, or mutation-specific targeting molecules including FLT3 inhibitors, IDH inhibitors, and monoclonal antibodies. Efforts to devise new, targeted therapy have included recent advances in methods for high-throughput genomic screening and the availability of computer-assisted techniques for the design of novel agents predicted to specifically inhibit mutant molecules involved in leukemogenesis. Crosstalk between the leukemia cells and the bone marrow microenvironment through cell surface molecules, such as the integrins αvβ3 and αvβ5, might influence drug response and AML progression. This review article focuses on current AML treatment options, new AML targeted therapies, the role of integrins in AML progression, and a potential therapeutic agent—integrin αvβ3 antagonist.

Similar content being viewed by others
Abbreviations
- AML:
-
Acute myeloid leukemia
- BCL-2:
-
B-cell lymphoma gene 2
- CAM:
-
Chick egg chorioallantoic membrane
- CDK:
-
Cyclin-dependent kinase
- c-KIT:
-
C-kit is a type of receptor tyrosine kinase, also called CD117
- c-FMS:
-
Proto-oncogene that codes for the MCSF (CSF1) receptor
- EGF:
-
Epidermal growth factor
- EGFR:
-
EGF receptor
- EMT:
-
Epithelial–mesenchymal transition
- FAK:
-
Focal adhesion kinase
- FGF:
-
Basic fibroblast growth factor
- FLT3:
-
FMS-like tyrosine kinase 3
- IDH:
-
Isocitrate dehydrogenase
- JAK-2:
-
Janus kinase-2
- LSD:
-
Lysine-specific demethylase
- MAPK:
-
Mitogen-activated protein kinase
- MDM2:
-
Mouse double minute chromosome 2
- miR:
-
MicroRNAs
- NDAT:
-
Nano-diamino-tetrac
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NPM:
-
Nucleophosmin
- P-bi-TAT:
-
Polyethylene glycol (PEG) covalently bonded with two bi-tri-azole tetraiodothyroacetic acid molecules
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed death-ligand 1
- PDGF:
-
Platelet-derived growth factor
- PEG:
-
Polyethylene glycol
- PI3K:
-
Phosphatidylinositol 3-kinase
- STAT:
-
Signal transducer and activator of transcription
- Tetrac:
-
Tetraiodothyroacetic acid
- TGFβ:
-
Transforming growth factor β
- TK:
-
Tyrosine kinase
- T4:
-
l-Thyroxine
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Greenberg PL, Gordeuk V, Issaragrisil S, Siritanaratkul N, Fucharoen S, Ribeiro RC. Major hematologic diseases in the developing world—new aspects of diagnosis and management of thalassemia, malarial anemia, and acute leukemia. Hematology Am Soc Hematol Educ Program. 2001:479–98.
Gocek E, Marcinkowska E. Differentiation therapy of acute myeloid leukemia. Cancers (Basel). 2011;3:2402–20.
American Cancer Society. Key statistics for acute myeloid leukemia (AML). 2019. https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html. Accessed 1 May 2019.
Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099–107.
Estey EH. Treatment of acute myeloid leukemia. Haematologica. 2009;94:10–6.
Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget. 2017;8:38022–43.
Johansen S, Brenner AK, Bartaula-Brevik S, Reikvam H, Bruserud Ø. The possible importance of β3 integrins for leukemogenesis and chemoresistance in acute myeloid leukemia. Int J Mol Sci. 2018;19:251.
Raab-Westphal S, Marshall JF, Goodman SL. Integrins as therapeutic targets: successes and cancers. Cancers (Basel). 2017;9:110.
Licht JD, Sternberg DW. The molecular pathology of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2005:137–42.
De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441.
Lagunas-Rangel FA, Chávez-Valencia V, Gómez-Guijosa MÁ, Cortes-Penagos C. Acute myeloid leukemia—genetic alterations and their clinical prognosis. Int J Hematol Oncol Stem Cell Res. 2017;11:328–39.
Zenonos K, Kyprianou K. RAS signaling pathways, mutations and their role in colorectal cancer. World J Gastrointest Oncol. 2013;5:97–101.
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74.
Montero J, Letai A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 2018;25:56–64.
Gibson CJ, Davids MS. BCL-2 antagonism to target the intrinsic mitochondrial pathway of apoptosis. Clin Cancer Res. 2015;21:5021–9.
Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010;2:a001107-a.
Kindle KB, Troke PJ, Collins HM, Matsuda S, Bossi D, Bellodi C, et al. MOZ-TIF2 inhibits transcription by nuclear receptors and p53 by impairment of CBP function. Mol Cell Biol. 2005;25:988–1002.
Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–66.
Liu S, Shen T, Huynh L, Klisovic MI, Rush LJ, Ford JL, et al. Interplay of RUNX1/MTG8 and DNA methyltransferase 1 in acute myeloid leukemia. Cancer Res. 2005;65:1277–84.
Stucki A, Rivier AS, Gikic M, Monai N, Schapira M, Spertini O. Endothelial cell activation by myeloblasts: molecular mechanisms of leukostasis and leukemic cell dissemination. Blood. 2001;97:2121–9.
Liu Z, Wang F, Chen X. Integrin αvβ3-targeted cancer therapy. Drug Dev Res. 2008;69:329–39.
Guo W, Giancotti FG. Integrin signaling during tumor progression. Nat Rev Mol Cell Biol. 2004;5:816–26.
Song X, Wei Z, Shaikh ZA. Requirement of ERα and basal activities of EGFR and Src kinase in Cd-induced activation of MAPK/ERK pathway in human breast cancer MCF-7 cells. Toxicol Appl Pharmacol. 2015;287:26–34.
Saif A, Kazmi SFA, Naseem R, Shah H, Butt MO. Acute myeloid leukemia: is that all there is? Cureus. 2018;10:e3198.
American Cancer Society. Chemotherapy for acute myeloid leukemia (AML). 2019. https://www.cancer.org/cancer/acute-myeloid-leukemia/treating/chemotherapy.html. Accessed 1 May 2019.
American Cancer Society. Risk factors for acute myeloid leukemia (AML). 2019. https://www.cancer.org/cancer/acute-myeloid-leukemia/causes-risks-prevention/risk-factors.html. Accessed 1 May 2019.
O’Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, et al. Acute myeloid leukemia, version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15:926–57.
Medinger M, Lengerke C, Passweg J. Novel prognostic and therapeutic mutations in acute myeloid leukemia. Cancer Genomics Proteomics. 2016;13:317–29.
Elsevier Inc. Clinical pharmacology: Cytarabine. 2019. https://www.clinicalkey.com/pharmacology/monograph/161?n=Cytarabine, ARA-C. Accessed 1 May 2019.
Elsevier Inc. Clinical pharmacology: Idarubicin. 2019. https://www.clinicalkey.com/pharmacology/monograph/305?n=Idarubicin. Accessed 1 May 2019.
Maurillo L, Buccisano F, Del Principe MI, Sarlo C, Di Caprio L, Ditto C, et al. Treatment of acute myeloid leukemia with 20–30% bone marrow blasts. Mediterr J Hematol Infect Dis. 2013;5:e2013032-e.
American Cancer Society. Cancer facts & Figs. 2017. 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed 1 May 2019.
Fathi AT, Karp JE. New agents in acute myeloid leukemia: beyond cytarabine and anthracyclines. Curr Oncol Rep. 2009;11:346–52.
• Lee LY, Hernandez D, Rajkhowa T, Smith SC, Raman JR, Nguyen B, et al. Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood. 2017;129:257–60 The inhibitory activity of gilteritinib against different forms of FLT3 mutations in leukemia cells was studied with immunoblotting. Gilteritinib showed significant inhibitor activity against different FLT3 mutations including the resistant mutations.
Liu T, Ivaturi V, Sabato P, Gobburu JV, Greer JM, Wright JJ, et al. Sorafenib dose recommendation in acute myeloid leukemia based on exposure-FLT3 relationship. Clin Transl Sci. 2018;11:435–43.
Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37.
Levis M, Allebach J, Tse K-F, Zheng R, Baldwin BR, Smith BD, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–91.
Wu M, Li C, Zhu X. FLT3 inhibitors in acute myeloid leukemia. J Hematol Oncol. 2018;11:133.
Levis M. Midostaurin approved for FLT3-mutated AML. Blood. 2017;129:3403–6.
Kampa-Schittenhelm KM, Heinrich MC, Akmut F, Dohner H, Dohner K, Schittenhelm MM. Quizartinib (AC220) is a potent second generation class III tyrosine kinase inhibitor that displays a distinct inhibition profile against mutant-FLT3, -PDGFRA and -KIT isoforms. Mol Cancer. 2013;12:19.
Kampa-Schittenhelm KM, Frey J, Haeusser LA, Illing B, Pavlovsky AA, Blumenstock G, et al. Crenolanib is a type I tyrosine kinase inhibitor that inhibits mutant KIT D816 isoforms prevalent in systemic mastocytosis and core binding factor leukemia. Oncotarget. 2017;8:82897–909.
Mathew NR, Baumgartner F, Braun L, O’Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018;24:282.
Lange A, Jaskula E, Lange J, Dworacki G, Nowak D, Simiczyjew A, et al. The sorafenib anti-relapse effect after alloHSCT is associated with heightened alloreactivity and accumulation of CD8+ PD-1+(CD279+) lymphocytes in marrow. PLoS One. 2018;13:e0190525.
Zhao W, Zhang T, Qu B, Wu X, Zhu X, Meng F, et al. Sorafenib induces apoptosis in HL60 cells by inhibiting Src kinase-mediated STAT3 phosphorylation. Anti-Cancer Drugs. 2011;22:79–88.
Feldmann F, Schenk B, Martens S, Vandenabeele P, Fulda S. Sorafenib inhibits therapeutic induction of necroptosis in acute leukemia cells. Oncotarget. 2017;8:68208.
Wander SA, Levis MJ, Fathi AT. The evolving role of FLT3 inhibitors in acute myeloid leukemia: quizartinib and beyond. Ther Adv Hematol. 2014;5:65–77.
DiNardo CD, Stone RM, Medeiros BC. Novel therapeutics in acute myeloid leukemia. Am Soc Clin Oncol Educ Book. 2017;37:495–503.
Sutamtewagul G, Vigil CE. Clinical use of FLT3 inhibitors in acute myeloid leukemia. Onco Targets Ther. 2018;11:7041–52.
Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7:165–76.
U.S. Food and Drug Administration. Novel drug approvals for 2018. 2018. https://www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm592464.htm. Accessed 1 May 2019.
Cucchi DG, Denys B, Kaspers GJ, Janssen JJ, Ossenkoppele GJ, de Haas V, et al. RNA-based FLT3-ITD allelic ratio is associated with outcome and ex vivo response to FLT3 inhibitors in pediatric AML. Blood. 2018;131:2485–9.
Mori M, Kaneko N, Ueno Y, Yamada M, Tanaka R, Saito R, et al. Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic activity in mouse models of FLT3 mutated acute myeloid leukemia. Investig New Drugs. 2017;35:556–65.
Short NJ, Kantarjian H, Ravandi F, Daver N. Emerging treatment paradigms with FLT3 inhibitors in acute myeloid leukemia. Ther Adv Hematol. 2019;10:2040620719827310.
Stone RM. Which new agents will be incorporated into frontline therapy in acute myeloid leukemia? Best Pract Res Clin Haematol. 2017;30:312–6.
Myers RA, Wirth S, Williams S, Kiel PJ. Enasidenib: An oral IDH2 inhibitor for the treatment of acute myeloid leukemia. J Adv Pract Oncol. 2018;9:435–40.
Abou Dalle I, DiNardo CD. The role of enasidenib in the treatment of mutant IDH2 acute myeloid leukemia. Ther Adv Hematol. 2018;9:163–73.
Tang A, Gao K, Chu L, Zhang R, Yang J, Zheng J. Aurora kinases: novel therapy targets in cancers. Oncotarget. 2017;8:23937–54.
Willems E, Dedobbeleer M, Digregorio M, Lombard A, Lumapat PN, Rogister B. The functional diversity of Aurora kinases: a comprehensive review. Cell Div. 2018;13:7.
Kim S-J, Jang JE, Cheong J-W, Eom J-I, Jeung H-K, Kim Y, et al. Aurora A kinase expression is increased in leukemia stem cells, and a selective Aurora A kinase inhibitor enhances Ara-C-induced apoptosis in acute myeloid leukemia stem cells. Korean J Hematol. 2012;47:178–85.
Yang Y, Shen Y, Li S, Jin N, Liu H, Yao X. Molecular dynamics and free energy studies on Aurora kinase A and its mutant bound with MLN8054: insight into molecular mechanism of subtype selectivity. Mol BioSyst. 2012;8:3049–60.
Wang X, Sinn AL, Pollok K, Sandusky G, Zhang S, Chen L, et al. Preclinical activity of a novel multiple tyrosine kinase and aurora kinase inhibitor, ENMD-2076, against multiple myeloma. Br J Haematol. 2010;150:313–25.
Melichar B, Adenis A, Lockhart AC, Bennouna J, Dees EC, Kayaleh O, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 2015;16:395–405.
Barr PM, Li H, Spier C, Mahadevan D, LeBlanc M, Ul Haq M, et al. Phase II intergroup trial of alisertib in relapsed and refractory peripheral T cell lymphoma and transformed mycosis fungoides: SWOG 1108. J Clin Oncol. 2015;33:2399–404.
Lowenberg B, Muus P, Ossenkoppele G, Rousselot P, Cahn JY, Ifrah N, et al. Phase 1/2 study to assess the safety, efficacy, and pharmacokinetics of barasertib (AZD1152) in patients with advanced acute myeloid leukemia. Blood. 2011;118:6030–6.
Kantarjian HM, Martinelli G, Jabbour EJ, Quintas-Cardama A, Ando K, Bay JO, et al. Stage I of a phase 2 study assessing the efficacy, safety, and tolerability of barasertib (AZD1152) versus low-dose cytosine arabinoside in elderly patients with acute myeloid leukemia. Cancer. 2013;119:2611–9.
Soncini C, Carpinelli P, Gianellini L, Fancelli D, Vianello P, Rusconi L, et al. PHA-680632, a novel Aurora kinase inhibitor with potent antitumoral activity. Clin Cancer Res. 2006;12:4080–9.
U.S. Food and Drug Administration. FDA approves venetoclax in combination for AML in adults. 2018. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm626499.htm. Accessed 1 May 2019.
Elsevier Inc. Clinical pharmacology: Venetoclax. 2019. https://www.clinicalkey.com/pharmacology/monograph/4844?n=Venetoclax. Accessed 1 May 2019.
AbbVie Inc. Highlights of prescribing information (venclexta). 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf. Accessed 1 May 2019.
Park MH, Hong JT. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5:15.
Annaloro C, Onida F, Saporiti G, Lambertenghi DG. Cancer stem cells in hematological disorders: current and possible new therapeutic approaches. Curr Pharm Biotechnol. 2011;12:217–25.
Shahshahan MA, Beckley MN, Jazirehi AR. Potential usage of proteasome inhibitor bortezomib (Velcade, PS-341) in the treatment of metastatic melanoma: basic and clinical aspects. Am J Cancer Res. 2011;1:913–24.
Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–9.
Ji Q, Ding Y-H, Sun Y, Zhang Y, Gao H-E, Song H-N, et al. Antineoplastic effects and mechanisms of micheliolide in acute myelogenous leukemia stem cells. Oncotarget. 2016;7:65012–23.
Darwish NH, Sudha T, Godugu K, Elbaz O, Abdelghaffar HA, Hassan EE, et al. Acute myeloid leukemia stem cell markers in prognosis and targeted therapy: potential impact of BMI-1, TIM-3 and CLL-1. Oncotarget. 2016;7:57811–20.
van Kemenade FJ, Raaphorst FM, Blokzijl T, Fieret E, Hamer KM, Satijn DP, et al. Coexpression of BMI-1 and EZH2 polycomb-group proteins is associated with cycling cells and degree of malignancy in B cell non-Hodgkin lymphoma. Blood. 2001;97:3896–901.
Mihara K, Chowdhury M, Nakaju N, Hidani S, Ihara A, Hyodo H, et al. Bmi-1 is useful as a novel molecular marker for predicting progression of myelodysplastic syndrome and patient prognosis. Blood. 2006;107:305–8.
Iwama A, Oguro H, Negishi M, Kato Y, Morita Y, Tsukui H, et al. Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity. 2004;21:843–51.
Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014;20:29–36.
Srinivasan M, Bharali DJ, Sudha T, Khedr M, Guest I, Sell S, et al. Downregulation of Bmi1 in breast cancer stem cells suppresses tumor growth and proliferation. Oncotarget. 2017;8:38731–42.
Elsevier Inc. Clinical pharmacology: Gemtuzumab ozogamicin. 2019. https://www.clinicalkey.com/pharmacology/monograph/2471?n=Gemtuzumab Ozogamicin. Accessed 1 May 2019.
•• Chin YT, Wei PL, Ho Y, Nana AW, Changou CA, Chen YR, et al. Thyroxine inhibits resveratrol-caused apoptosis by PD-L1 in ovarian cancer cells. Endocr Relat Cancer. 2018;25:533–45 Authors demonstrated the potential impact of thyroid hormones on cancer apoptosis. T4 inhibited COX-2-dependent apoptosis in ovarian cancer cells by retaining inducible COX-2 with PD-L1 in the cytoplasm. These findings provide new insights into the effect of T4 antagonizing factors on cancer properties.
Cremaschi GA, Cayrol F, Sterle HA, Diaz Flaque MC, Barreiro Arcos ML. Thyroid hormones and their membrane receptors as therapeutic targets for T cell lymphomas. Pharmacol Res. 2016;109:55–63.
Davis PJ, Sudha T, Lin HY, Mousa SA. Thyroid hormone, hormone analogs, and angiogenesis. Compr Physiol. 2015;6:353–62.
Lin HY, Chin YT, Yang YC, Lai HY, Wang-Peng J, Liu LF, et al. Thyroid hormone, cancer, and apoptosis. Compr Physiol. 2016;6:1221–37.
Pinto M, Soares P, Ribatti D. Thyroid hormone as a regulator of tumor induced angiogenesis. Cancer Lett. 2011;301:119–26.
Shinderman-Maman E, Cohen K, Weingarten C, Nabriski D, Twito O, Baraf L, et al. The thyroid hormone-αvβ3 integrin axis in ovarian cancer: regulation of gene transcription and MAPK-dependent proliferation. Oncogene. 2016;35:1977–87.
Bailey EB, Tantravahi SK, Poole A, Agarwal AM, Straubhar AM, Batten JA, et al. Correlation of degree of hypothyroidism with survival outcomes in patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor receptor tyrosine kinase inhibitors. Clin Genitourin Cancer. 2014;ed2015:e131–7.
Cristofanilli M, Yamamura Y, Kau SW, Bevers T, Strom S, Patangan M, et al. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer. 2005;103:1122–8.
Nelson M, Hercbergs A, Rybicki L, Strome M. Association between development of hypothyroidism and improved survival in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:1041–6.
Schmidinger M, Vogl UM, Bojic M, Lamm W, Heinzl H, Haitel A, et al. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer. 2011;117:534–44.
Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12:111–21.
Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–70.
Davis PJ, Glinsky GV, Lin H-Y, Leith JT, Hercbergs A, Tang H-Y, et al. Cancer cell gene expression modulated from plasma membrane integrin αvβ3 by thyroid hormone and nanoparticulate tetrac. Front Endocrinol (Lausanne). 2015;5:240.
Yi H, Zeng D, Shen Z, Liao J, Wang X, Liu Y, et al. Integrin alphavbeta3 enhances β-catenin signaling in acute myeloid leukemia harboring Fms-like tyrosine kinase-3 internal tandem duplication mutations: implications for microenvironment influence on sorafenib sensitivity. Oncotarget. 2016;7:40387–97.
Gruszka AM, Valli D, Restelli C, Alcalay M. Adhesion deregulation in acute myeloid leukemia. Cells. 2019;8:66.
Shi S, Zhou M, Li X, Hu M, Li C, Li M, et al. Synergistic active targeting of dually integrin alphavbeta3/CD44-targeted nanoparticles to B16F10 tumors located at different sites of mouse bodies. J Control Release. 2016;235:1–13.
Weingarten C, Jenudi Y, Tshuva RY, Moskovich D, Alfandari A, Hercbergs A, et al. The interplay between epithelial–mesenchymal transition (EMT) and the thyroid hormones–αvβ3 axis in ovarian cancer. Horm Cancer. 2018;9:22–32.
Zhang P, Chen L, Song Y, Li X, Sun Y, Xiao Y, et al. Tetraiodothyroacetic acid and transthyretin silencing inhibit pro-metastatic effect of L-thyroxin in anoikis-resistant prostate cancer cells through regulation of MAPK/ERK pathway. Exp Cell Res. 2016;347:350–9.
Abou-El-Naga AM, Mutawa G, El-Sherbiny IM, Mousa SA. Activation of polymeric nanoparticle intracellular targeting overcomes chemodrug resistance in human primary patient breast cancer cells. Int J Nanomedicine. 2018;13:8153–64.
•• Sudha T, Bharali DJ, Yalcin M, Darwish NH, Debreli Coskun M, Keating KA, et al. Targeted delivery of paclitaxel and doxorubicin to cancer xenografts via the nanoparticle of nano-diamino-tetrac. Int J Nanomedicine. 2017;12:1305–15 Authors discuss the potential impact of therapies that target integrin αvβ3. They demonstrated the feasibility of chemotherapy delivery using a nanoparticle system that achieved higher intratumoral concentrations and improved antitumor efficacy of chemotherapies than via the conventional administration route of these agents.
Mousa SA, Glinsky GV, Lin HY, Ashur-Fabian O, Hercbergs A, Keating KA, et al. Contributions of thyroid hormone to cancer metastasis. Biomedicines. 2018;6:89.
Cai X, Zhu H, Li Y. Pkczeta, MMP2 and MMP9 expression in lung adenocarcinoma and association with a metastatic phenotype. Mol Med Rep. 2017;16:8301–6.
Hong M, Cheng H, Song L, Wang W, Wang Q, Xu D, et al. Wogonin suppresses the activity of matrix metalloproteinase-9 and inhibits migration and invasion in human hepatocellular carcinoma. Molecules. 2018;23:384.
Tauro M, Lynch CC. Cutting to the chase: how matrix metalloproteinase-2 activity controls breast-cancer-to-bone metastasis. Cancers (Basel). 2018;10:185.
Cohen K, Flint N, Shalev S, Erez D, Baharal T, Davis PJ, et al. Thyroid hormone regulates adhesion, migration and matrix metalloproteinase 9 activity via αvβ3 integrin in myeloma cells. Oncotarget. 2014;5:6312–22.
Bridoux A, Khan RA, Chen C, Cheve G, Cui H, Dyskin E, et al. Design, synthesis, and biological evaluation of bifunctional thyrointegrin inhibitors: new anti-angiogenesis analogs. J Enzyme Inhib Med Chem. 2011;26:871–82.
Mousa SA, Mousa AS. Angiogenesis inhibitors: current & future directions. Curr Pharm Des. 2004;10:1–9.
Davis PJ, Mousa SA, Lin HY. Tetraiodothyroacetic acid (tetrac), integrin αvβ3 and disabling of immune checkpoint defense. Future Med Chem. 2018;10:1637–9.
Mousa SA, Rajabi M, inventors. Non-cleaveable polymer conjugated with αvβ3 integrin thyroid antagonists. USA patent 10, 201,616. 2019.
Rajabi M, Godugu K, Sudha T, Bharali DJ, Mousa SA. Triazole modified tetraiodothyroacetic acid conjugated to polyethylene glycol: High affinity thyrointegrin αvβ3 antagonist with potent anticancer activities in glioblastoma multiforme. Bioconj Chem. 2019;30:3087-97.
Funding
There is no funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shaheedul A. Sami declares that he has no conflict of interest.
Noureldien H. E. Darwish declares that he has no conflict of interest.
Amanda N. M. Barile declares that she has no conflict of interest.
Shaker A. Mousa was issued a patent that is owned by NanoPharmaceuticals LLC, and he owns stock in NanoPharmaceuticals LLC, which is developing anticancer drugs.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Sami, S.A., Darwish, N.H.E., Barile, A.N.M. et al. Current and Future Molecular Targets for Acute Myeloid Leukemia Therapy. Curr. Treat. Options in Oncol. 21, 3 (2020). https://doi.org/10.1007/s11864-019-0694-6
Published:
DOI: https://doi.org/10.1007/s11864-019-0694-6