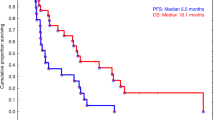
Opinion statement
Primary plasma cell leukemia (PPCL) is an aggressive and rare variant of multiple myeloma (MM), characterized by peculiar adverse clinical and biological features. Though the poor outcome of PPCL has been slightly improved by novel treatments during the last 10 years, due to the limited number of available studies in this uncommon disease, optimal therapy remains a classic unmet clinical need. Anyway, in the real-life practice, induction with a bortezomib-based three-drug combination, including dexamethasone and, possibly, lenalidomide, or, alternatively, thalidomide, cyclophosphamide, or doxorubicin, is a reasonable first-line option. This approach may be particularly advisable for patients with adverse cytogenetics, hyperleucocytosis, and rapidly progressive disease, in whom a fast response is required, or for those with suboptimal renal function, where, however, lenalidomide should be used with caution until renal activity is restored. In younger subjects, leukemia/lymphoma-like more intensive regimens, including hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone or continue-infusion cisplatin, doxorubicin, cyclophosphamide, and etoposide, may be also combined with bortezomib +/- thalidomide. Treatment must be started immediately after a diagnosis of PPCL is made to avoid the risk of irreversible disease complications and, in such a context, the prevention of tumor lysis syndrome is mandatory. In patients eligible for autologous stem cell transplantation (AuSCT), other alkylating agents, in particular melphalan, should be initially avoided in order to allow adequate collections of CD34+ peripheral blood stem cells (PBSC). A combination of lenalidomide and dexamethasone may be a valuable alternative option to manage older or unfit patients or those with slower disease evolution or with signs of neuropathy, contraindicating the use of bortezomib. Patients not suitable for transplant procedures should continue the treatment, if a response occurs and if tolerated, considering the possibility of a prolonged maintenance therapy. AuSCT should be pursued in all eligible patients less than 65 years old who achieve a significant response after a short course of induction treatment. PBSC collection should reach a threshold of at least 5 × 106 CD34+ PBSC/kg using cyclophosphamide plus G-CSF and adding the mobilizing agent plerixafor, if necessary. High-dose melphalan (HDM) (200 or 140 mg/m2, according to age and renal function) remains the preferable conditioning regimen. A second AuSCT should be always considered, even in patients achieving complete response (CR) after the first AuSCT, as the short progression-free survival (PFS) generally seen in PPCL suggests the persistence of a relevant burden of residual disease; this provides a strong rationale for the use of post-transplantation therapies in PPCL to improve depth of response, to maintain remission, and, possibly, to increase survival, though consolidation and/or maintenance strategies with novel agents, whose efficacy has been well demonstrated in MM, have not been still extensively evaluated in PPCL. The search of a suitable donor should start as soon as possible and an allogeneic stem cell transplant (AlloSCT) with a myeloablative conditioning (MAC) regimen discussed with younger patients responsive to induction therapy and with poor prognostic parameters at diagnosis. A sequence of AuSCT followed by reduced intensity conditioning (RIC) or non-myeloablative (NMA) AlloSCT may be considered in selected cases. Salvage therapies for relapsed/refractory disease, especially using new drugs not employed at diagnosis, are sometimes effective in the short term, but a rapid relapse is still generally the rule; AlloSCT in relapsed and eligible patients with sensitive disease after salvage treatments is, therefore, recommended.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Han X, Kilfoy B, Zheng T, Holford TR, Zhu C, Zhu Y, et al. Lymphoma survival patterns by WHO subtype in the United States, 1973–2003. Cancer Causes Control. 2008;19:841–58.
Ramsingh G, Mehan P, Luo J, Vij R, Morgensztern D. Primary plasma cell leukemia: a Surveillance, Epidemiology, and End Results database analysis between 1973 and 2004. Cancer. 2009;115:5734–9.
Gonsalves WI, Rajkumar SV, Go RS, Dispenzieri A, Gupta V, Singh PP, et al. Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood. 2014;124:907–12. The most important and updated registry study for PPCL, which demonstrates a moderate, but significant improvement in survival after the introduction of novel agents as first line treatment in the clinical practice.
Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia. Report on 17 cases. Arch Intern Med. 1974;133:813–8.
FernándezdeLarrea C, Kyle RA, Durie BGM, Ludwig H, Usmani S, Vesole DH, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780–91. An extensive review with international consensus on diagnostic and response criteria and treatment recommendations for PCL.
Jelinek T, Kryukov F, Rihova L, Hajek R. Plasma cell leukemia: from biology to treatment. Eur J Haematol. 2015;95:16–26. The most recent and updated review of clinical and biological features of PCL.
An G, Qin X, Acharya C, Xu Y, Deng S, Shi L, et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann Hematol. 2015;94:257–64.
Gonsalves WI, Rajkumar SV, Gupta V, Morice WG, Timm MM, Singh PP, et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: implications for redefining high-risk myeloma. Leukemia. 2014;28:2060–5.
Cha CH, Park CJ, Huh JR, Chi HS, Suh CW, Kang YK. Significantly better prognosis for patients with primary plasma cell leukemia than for patients with secondary plasma cell leukemia. Acta Haematol. 2007;118:178–82.
Tiedemann RE, Gonzalez-Paz N, Kyle RA, Santana-Davila R, Price-Troska T, Van Wier SA, et al. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008;22:1044–52.
Lebovic D, Zhang L, Alsina M, Nishihori T, Shain KH, Sullivan D, et al. Clinical outcomes of patients with plasma cell leukemia in the era of novel therapies and hematopoietic stem cell transplantation strategies: a single-institution experience. Clin Lymphoma Myeloma Leuk. 2011;11:507–11.
Bladé J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am. 1999;13:1259–72.
Dimopoulos MA, Palumbo A, Delasalle KB, Alexanian R. Primary plasma cell leukaemia. Br J Haematol. 1994;88:754–9.
García-Sanz R, Orfão A, González M, Tabernero MD, Bladé J, Moro MJ, et al. Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics. Blood. 1999;93:1032–7.
Noel P, Kyle RA. Plasma cell leukemia: an evaluation of response to therapy. Am J Med. 1987;83:1062–8.
Liedtke M, Medeiros BC. Plasma cell leukemia: concepts and management. Expert Rev Hematol. 2010;3:543–9.
Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724–34.
Musto P, Pagano L, Petrucci MT, Morabito F, Caravita T, Di Raimondo F, et al. Primary plasma cell leukemia in the era of new drugs: has something changed? Crit Rev Oncol Hematol. 2012;82:141–9.
van de Donk NWCJ, Lokhorst HM, Anderson KC, Richardson PG. How I treat plasma cell leukemia. Blood. 2012;120:2376–89.
Nishihori T, Abu Kar SM, Baz R, Alsina M, Harousseau J-L, Kharfan-Dabaja MA. Therapeutic advances in the treatment of primary plasma cell leukemia: a focus on hematopoietic cell transplantation. Biol Blood Marrow Transplant Elsevier Ltd. 2013;19:1144–51. A comprehensive description of PPCL, with practical recommendations for the management and a major focus on the role of autologous and allogeneic transplantation in this disorder, indicating that these procedures may be effective in PPCL, but a lower level than in multiple myeloma.
Kraj M, Kopeć-Szlęzak J, Pogłód R, Kruk B. Flow cytometric immunophenotypic characteristics of 36 cases of plasma cell leukemia. Leuk Res. 2011;35:169–76.
Avet-Loiseau H, Roussel M, Campion L, Leleu X, Marit G, Jardel H, et al. Cytogenetic and therapeutic characterization of primary plasma cell leukemia: the IFM experience. Leukemia. Nature Publishing Group 2012;26:158–9.
Simeon V, Todoerti K, La Rocca F, Caivano A, Trino S, Lionetti M, et al. Molecular classification and pharmacogenetics of primary plasma cell leukemia: an initial approach toward precision medicine. Int J Mol Sci. 2015;16:17514–34. A comprehensive and updated review of molecular characteristics and pharmacogenetics of multiple myeloma and PPCL.
Chiecchio L, Protheroe RKM, Ibrahim AH, Cheung KL, Rudduck C, Dagrada GP, et al. Deletion of chromosome 13 detected by conventional cytogenetics is a critical prognostic factor in myeloma. Leukemia. 2006;20:1610–7.
Chiecchio L, Dagrada GP, White HE, Towsend MR, Protheroe RKM, Kan LC, et al. Frequent upregulation of MYC in plasma cell leukemia. Genes Chromosom Cancer. 2009;48:624–36.
Mosca L, Musto P, Todoerti K, Barbieri M, Agnelli L, Fabris S, et al. Genome-wide analysis of primary plasma cell leukemia identifies recurrent imbalances associated with changes in transcriptional profiles. Am J Hematol. 2013;88:16–23. This is the first extensive genomic analysis performed in a prospective series of PPCL by means of FISH, single-nucleotide polymorphism (SNP) and gene expression profiling (GEP).
Avet-Loiseau H, Daviet A, Brigaudeau C, Callet-Bauchu E, Terré C, Lafage-Pochitaloff M, et al. Cytogenetic, interphase, and multicolor fluorescence in situ hybridization analyses in primary plasma cell leukemia: a study of 40 patients at diagnosis, on behalf of the Intergroupe Francophone du Myelome and the Groupe Francais de Cytogenetique Hematolo. Blood. 2001;97:822–5.
Pagano L, Valentini CG, De Stefano V, Venditti A, Visani G, Petrucci MT, et al. Primary plasma cell leukemia: a retrospective multicenter study of 73 patients. Ann Oncol. 2011;22:1628–35. A large retrospective study in PPCL with relevant information about clinical outcome according to different treatments employed (conventional chemotherapy, novel agents, and stem cell transplantation).
Chang H, Qi X, Yeung J, Reece D, Xu W, Patterson B. Genetic aberrations including chromosome 1 abnormalities and clinical features of plasma cell leukemia. Leuk Res. 2009;33:259–62.
Chang H, Sloan S, Li D, Patterson B. Genomic aberrations in plasma cell leukemia shown by interphase fluorescence in situ hybridization. Cancer Genet Cytogenet. 2005;156:150–3.
Musto P, Simeon V, Martorelli MC, Petrucci MT, Cascavilla N, Di Raimondo F, et al. Lenalidomide and low-dose dexamethasone for newly diagnosed primary plasma cell leukemia. Leukemia. 2014;28:222–5. The first prospective clinical trial in PPCL, documenting efficacy and safety of lenalidomide in combination with dexamethasone (Rd) as frontline treatment and of lenalidomide as maintenance therapy; in this study, a significant improvement of survival in patients undergoing stem cell transplantation after Rd induction was obtained.
Usmani SZ, Rodriguez-Otero P, Bhutani M, Mateos M-V, Miguel JS. Defining and treating high-risk multiple myeloma. Leukemia. 2015;29:2119–25.
Usmani SZ, Nair B, Qu P, Hansen E, Zhang Q, Petty N, et al. Primary plasma cell leukemia: clinical and laboratory presentation, gene-expression profiling and clinical outcome with Total Therapy protocols. Leukemia. 2012;26:2398–405.
Todoerti K, Agnelli L, Fabris S, Lionetti M, Tuana G, Mosca L, et al. Transcriptional characterization of a prospective series of primary plasma cell leukemia revealed signatures associated with tumor progression and poorer outcome. Clin Cancer Res. 2013;19:3247–58. A genomic study of PPCL revealing specific gene expression profile signatures correlated with diagnostic features and clinical outcome.
Lionetti M, Musto P, Di Martino MT, Fabris S, Agnelli L, Todoerti K, et al. Biological and clinical relevance of miRNA expression signatures in primary plasma cell leukemia. Clin Cancer Res. 2013;19:3130–42. The first evidence of the potential role of miRNA in the pathobiology of PPCL, with possible relationships with response to therapy and survival.
Cifola I, Lionetti M, Pinatel E, Todoerti K, Mangano E, Pietrelli A, et al. Whole-exome sequencing of primary plasma cell leukemia discloses heterogeneous mutational patterns. Oncotarget. 2015;6:17543–58. This is the first whole-exome sequencing screen in PPCL, evidencing remarkable genetic heterogeneity of mutational patterns.
Lionetti M, Barbieri M, Todoerti K, Agnelli L, Marzorati S, Fabris S, et al. Molecular spectrum of BRAF, NRAS and KRAS gene mutations in plasma cell dyscrasias: implication for MEK-ERK pathway activation. Oncotarget. 2015;15:24205–17.
Bernasconi C, Castelli G, Pagnucco G, Brusamolino E. Plasma cell leukemia: a report on 15 patients. Eur J Haematol Suppl. 1989;51:76–83.
Costello R, Sainty D, Bouabdallah R, Fermand JP, Delmer A, Diviné M, et al. Primary plasma cell leukaemia: a report of 18 cases. Leuk Res. 2001;25:103–7.
Vela-Ojeda J, García-Ruiz Esparza MA, Rosas-Cabral A, Padilla-González Y, García-Chávez J, Tripp-Villanueva F, et al. Intermediate doses of melphalan and dexamethasone are better than vincristine, adriamycin, and dexamethasone (VAD) and polychemotherapy for the treatment of primary plasma cell leukemia. Ann Hematol. 2002;81:362–7.
Jiménez-Zepeda VH, Domínguez VJ. Plasma cell leukemia: a rare condition. Ann Hematol. 2006;85:263–7.
Colović M, Janković G, Suvajdzić N, Milić N, Dordević V, Janković S. Thirty patients with primary plasma cell leukemia: a single center experience. Med Oncol. 2008;25:154–60.
Peijing Q, Yan X, Yafei W, Dehui Z, Zengjun L, Junyuan Q, et al. A retrospective analysis of thirty-one cases of plasma cell leukemia from a single center in China. Acta Haematol. 2009;121:47–51.
Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125:3076–85.
Shah N, Callander N, Ganguly S, Gul Z, Hamadani M, Costa L, et al. Hematopoietic stem cell transplantation for multiple myeloma: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21:1155–66.
Saccaro S, Fonseca R, Veillon DM, Cotelingam J, Nordberg ML, Bredeson C, et al. Primary plasma cell leukemia: report of 17 new cases treated with autologous or allogeneic stem-cell transplantation and review of the literature. Am J Hematol. 2005;78:288–94.
Drake MB, Iacobelli S, van Biezen A, Morris C, Apperley JF, Niederwieser D, et al. Primary plasma cell leukemia and autologous stem cell transplantation. Haematologica. 2010;95:804–9.
Mahindra A, Kalaycio ME, Vela-Ojeda J, Vesole DH, Zhang M-J, Li P, et al. Hematopoietic cell transplantation for primary plasma cell leukemia: results from the Center for International Blood and Marrow Transplant Research. Leukemia. 2012;26:1091–7. A large registry study with comparison between autologous and allogeneic transplant procedures in PPCL, showing that (i) autologous transplantation can improve outcome of patients, although results are markedly inferior to those achieved in patients with multiple myeloma, and (ii) no significant benefit in survival occurs after allo-transplantation.
Landsburg DJ, Vogl DT, Plastaras JP, Stadtmauer EA. Melphalan/total body irradiation-conditioned myeloablative allogeneic hematopoietic cell transplantation for patients with primary plasma cell leukemia. Clin Lymphoma Myeloma Leuk. 2014;14:e225–8.
Morris C, Iacobelli S, Gahrton G, Garderet L, Drake M, van Biezen A, et al. Has allogeneic transplantation a role in the management of plasma cell leukaemia? A study on behalf of the Myeloma Subcomittee of the Chronic Leukaemia Working Party of the EBMT. Blood (ASH Annual Meeting Abstract). 2011. Abstract 2008. Available from: https://ash.confex.com/ash/2011/webprogram/Paper43375.html.
Bensinger WI. Allogeneic stem cell transplantation for multiple myeloma. Hematol Oncol Clin North Am. 2014;28:891–902.
Laubach J, Garderet L, Mahindra A, Gahrton G, Caers J, Sezer O, et al. Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia. 2015; Dec 29. doi: 10.1038/leu.2015.356. PMID: 26710887
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–8.
Petrucci MT, Martini V, Levi A, Gallucci C, Palumbo G, Del Bianco P, et al. Thalidomide does not modify the prognosis of plasma cell leukemia patients: experience of a single center. Leuk Lymphoma. 2007;48:180–2.
Bauduer F. Efficacy of thalidomide in the treatment of VAD-refractory plasma cell leukaemia appearing after autologous stem cell transplantation for multiple myeloma. Br J Haematol. 2002;117:996–7.
Tsiara S, Chaidos A, Kapsali H, Tzouvara E, Bourantas KL. Thalidomide administration for the treatment of resistant plasma cell leukemia. Acta Haematol. 2003;109:153–5.
Brück P, Mousset S, Bühme A, Hoelzer D, Atta J. Nonsecretory primary plasma cell leukemia with good response to thalidomide-based treatment. Int J Hematol. 2007;86:66–8.
Johnston RE, Abdalla SH. Thalidomide in low doses is effective for the treatment of resistant or relapsed multiple myeloma and for plasma cell leukaemia. Leuk Lymphoma. 2002;43:351–4.
Wöhrer S, Ackermann J, Baldia C, Seidl S, Raderer M, Simonitsch I, et al. Effective treatment of primary plasma cell leukemia with thalidomide and dexamethasone—a case report. Hematol J. 2004;5:361–3.
Sher T, Miller KC, Deeb G, Lee K, Chanan-Khan A. Plasma cell leukaemia and other aggressive plasma cell malignancies. Br J Haematol. 2010;150:418–27.
Abe M, Yokoyama H, Tohmiya Y, Okitsu Y, Ohguchi H, Kohata K, et al. Plasma cell leukemia maintaining complete remission by syngeneic stem cell transplantation combined with low-dose thalidomide maintenance therapy. Intern Med. 2009;48:1833–5.
D’Arena G, Valentini CG, Pietrantuono G, Guariglia R, Martorelli MC, Mansueto G, et al. Frontline chemotherapy with bortezomib-containing combinations improves response rate and survival in primary plasma cell leukemia: a retrospective study from GIMEMA Multiple Myeloma Working Party. Ann Oncol. 2012;23:1499–502. A large retrospective multicenter study demonstrating the efficacy of bortezomib-based treatments in PPCL.
Katodritou E, Terpos E, Kelaidi C, Kotsopoulou M, Delimpasi S, Kyrtsonis M-C, et al. Treatment with bortezomib-based regimens improves overall response and predicts for survival in patients with primary or secondary plasma cell leukemia: analysis of the Greek myeloma study group. Am J Hematol. 2014;89:145–50. Retrospective analysis including 25 PPCL in which bortezomib-treated patients had a better survival than those receiving conventional therapies, regardless of additional autologous transplant rescue or established high risk features.
Ballanti S, Mastrodicasa E, Bolli N, Lotti F, Capolsini I, Berchicci L, et al. Sustained ventricular tachycardia in a thalidomide-treated patient with primary plasma-cell leukemia. Nat Clin Pract Oncol. 2007;4:722–5.
Pretz J, Medeiros BC. Thalidomide-induced pneumonitis in a patient with plasma cell leukemia: no recurrence with subsequent lenalidomide therapy. Am J Hematol. 2009;84:698–9.
Benson DM, Smith MK. Effectiveness of lenalidomide (Revlimid) for the treatment of plasma cell leukemia. Leuk Lymphoma. 2007;48:1423–5.
Musto P, Pietrantuono G, Guariglia R, Villani O, Martorelli MC, D’Auria F, et al. Salvage therapy with lenalidomide and dexamethasone in relapsed primary plasma cell leukemia. Leuk Res. 2008;32:1637–8.
Guglielmelli T, Merlini R, Giugliano E, Saglio G. Lenalidomide, melphalan, and prednisone association is an effective salvage therapy in relapsed plasma cell leukaemia. J Oncol. 2009;2009:867380.
Olivieri A, Attolico I, Cimminiello M, Discepoli G, Cifarelli RA. Lenalidomide can induce graft versus leukemia effect in primary plasma cell leukemia: a case report. Leuk Res. 2009;33:e191–3.
Mele G, Coppi MR, Guaragna G, Spina A, Melpignano A. Response to pomalidomide plus fixed low-dose dexamethasone in a case of secondary plasma cell leukaemia. Leuk Res. 2016;40:30–2.
Esparís-Ogando A, Alegre A, Aguado B, Mateo G, Gutiérrez N, Bladé J, et al. Bortezomib is an efficient agent in plasma cell leukemias. Int J Cancer. 2005;114:665–7.
Finnegan DPJ, Kettle P, Drake M, Matthews C, Alexander HD, Popat R, et al. Bortezomib is effective in primary plasma cell leukemia. Leuk Lymphoma. 2006;47:1670–3.
Ataergin S, Arpaci F, Kaya A, Kaya T, Gunhan O. VAD combination chemotherapy followed by bortezomib may be an effective treatment in secondary plasma cell leukemia. Am J Hematol. 2006;81:987–8.
Grassinger J, Südhoff T, Andreesen R, Hennemann B. Complete remission and successful stem cell mobilization after treatment of refractory plasma cell leukemia with bortezomib. Ann Hematol. 2006;85:132–3.
Musto P, Rossini F, Gay F, Pitini V, Guglielmelli T, D’Arena G, et al. Efficacy and safety of bortezomib in patients with plasma cell leukemia. Cancer. 2007;109:2285–90.
Ali R, Beksac M, Ozkalemkas F, Ozkocaman V, Ozkan A, Ozcelik T, et al. Efficacy of bortezomib in combination chemotherapy on secondary plasma cell leukemia. Leuk Lymphoma. 2007;48:1426–8.
Kim SJ, Kim J, Cho Y, Seo BK, Kim BS. Combination chemotherapy with bortezomib, cyclophosphamide and dexamethasone may be effective for plasma cell leukemia. Jpn J Clin Oncol. 2007;37:382–4.
Krüger WH, Kiefer T, Schüler F, Lotze C, Busemann C, Dölken G. Complete remission and early relapse of refractory plasma cell leukemia after bortezomib induction and consolidation by HLA-mismatched unrelated allogeneic stem cell transplantation. Onkologie. 2007;30:193–5.
Capalbo S, Chiefa A, Delia M, Diomede D, Liso V. Effective combination therapy of bortezomib and dexamethasone for a plasma cell leukemia patient with multiple osteolytic lesions and extramedullary involvement. Acta Oncol (Madr). 2007;46:262–4.
Al-Nawakil C, Tamburini J, Bardet V, Chapuis N, Bourry E, Roux C, et al. Bortezomib, doxorubicin and dexamethasone association is an effective option for plasma cell leukemia induction therapy. Leuk Lymphoma. 2008;49:2012–4.
Telek B, Méhes L, Batár P, Kiss A, Udvardy M. Effective PAD (bortezomib, doxorubicine, dexamethasone) treatment of a patient with plasma cell leukaemia developed after autologous stem cell transplantation. Orv Hetil. 2008;149:1957–9.
Bernardeschi P, Pirrotta MT, Montenora I, Giustarini G, Ferreri MI, Simi P, et al. Clonal evolution at leukemic relapse of multiple myeloma (secondary plasma cell leukemia) responding to re-treatment with bortezomib-based therapy. a case record. Leuk Res. 2010;34:e104–5.
Libby E, Candelaria-Quintana D, Moualla H, Abdul-Jaleel M, Rabinowitz I. Durable complete remission of primary plasma cell leukemia with the bortezomib plus melphalan and prednisone (VMP) regimen. Am J Hematol. 2010;85:733–4.
Mele G, Pinna S, Melpignano A, Quarta G. Retrospective case series of three patients with plasma cell leukemia treated with bortezomib-based regimens. Clin Ther. 2010;32:915–9.
Jaskiewicz AD, Herrington JD, Wong L. Tumor lysis syndrome after bortezomib therapy for plasma cell leukemia. Pharmacotherapy. 2005;25:1820–5.
Katodritou E, Verrou E, Gastari V, Hadjiaggelidou C, Terpos E, Zervas K. Response of primary plasma cell leukemia to the combination of bortezomib and dexamethasone: do specific cytogenetic and immunophenotypic characteristics influence treatment outcome? Leuk Res. 2008;32:1153–6.
Gozzetti A, Musto P, Defina M, D’Auria F, Papini G, Steduto T, et al. Efficacy of bortezomib, lenalidomide and dexamethasone (VRD) in secondary plasma cell leukaemia. Br J Haematol. 2012;157:497–8.
Ueda S, Kubo M, Matsuura N, Matsunaga H, Kataoka S, Maeda T, et al. Stringent complete remission of primary plasma cell leukemia with reduced-dose bortezomib, lenalidomide and dexamethasone: a case report and review of the literature. Intern Med. 2013;52:1235–8.
Jimenez-Zepeda VH, Reece DE, Trudel S, Chen C, Tiedemann R, Kukreti V. Lenalidomide (Revlimid), bortezomib (Velcade) and dexamethasone for the treatment of secondary plasma cell leukemia. Leuk Lymphoma. 2015;56:232–5.
Tamura S, Koyama A, Shiotani C, Kurihara T, Nishikawa A, Okamoto Y, et al. Successful bortezomib/dexamethasone induction therapy with lenalidomide in an elderly patient with primary plasma cell leukemia complicated by renal failure and pulmonary hypertension. Intern Med. 2014;53:1171–5.
Royer B, Magrangeas F, Lioure B, Fermand J-P, Hulin C, Karlin L, et al. First large prospective study for patients with primary plasma cell leukemia: bortezomib-doxorubicin-dexamethasone/bortezomib-cyclophosphamide-dexamethasone regimens as induction before stem cell transplantation followed by consolidation with lenalidomide. Blood (ASH Annual Meeting Abstract). 2013. Abstract 761. Available from: http://www.myelomabeacon.com/tag/ash-2013-plasma-cell-leukemia/.Interim analysis of a prospective, phase 2 study with bortezomib during induction, followed by either double autologous transplantation and bortezomib plus lenalidomide as consolidation/maintenance therapy or tandem sequence of autologous followed by reduced intensity allogeneic transplantation, in younger patients with newly diagnosed PPCL.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–52.
Talamo G, Dolloff NG, Sharma K, Zhu J, Malysz J. Clinical features and outcomes of plasma cell leukemia: a single-institution experience in the era of novel agents. Rare Tumors. 2012;4:e39.
Iriuchishima H, Ozaki S, Konishi J, Matsumoto M, Murayama K, Nakamura F, et al. Primary plasma cell leukemia in the era of novel agents: a multicenter study of the Japanese Society of Myeloma. Acta Haematol. 2016;135:113–21.
Musto P. Novel agents for the treatment of primary plasma-cell leukemia: lights and shadows. Acta Haematol. 2016;135:110–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Pellegrino Musto has received honoraria from Celgene, Janssen, Bristol-Myers Squibb, Sanofi, and Amgen for development of educational presentations including service on speakers’ bureaus.
The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Musto, P., Simeon, V., Todoerti, K. et al. Primary Plasma Cell Leukemia: Identity Card 2016. Curr. Treat. Options in Oncol. 17, 19 (2016). https://doi.org/10.1007/s11864-016-0392-6
Published:
DOI: https://doi.org/10.1007/s11864-016-0392-6