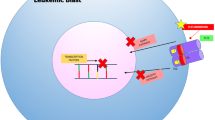
Opinion statement
Traditional cytotoxic chemotherapy is effective at temporizing AML in the majority of patients but cures a small minority. Thus, enrollment in clinical trials remains a recommended approach for nearly all patients. While signal transduction inhibition is a promising area to advance AML therapy, no agent as monotherapy has demonstrated obvious clinical benefit over traditional cytotoxic chemotherapy. Tipifarnib is perhaps an exception as it is the only signal transduction inhibitor in AML that reproducibly shows clinical benefit using traditional chemotherapy response criteria. Due to toxicity and low response rates, however, the potential advantages of tipifarnib over either traditional cytotoxic chemotherapy or best supportive care alone await confirmation from phase III studies. Available data suggest that combining signal transduction inhibitors with chemotherapy will improve response rates. Clinical trials to test this hypothesis are ongoing using various agents directed against targets such as FLT3, ras/raf/MAPK, mTOR, KIT, and VEGF, but the optimal approach is yet to be defined. Similarly unclear is the benefit of a potent specific kinase inhibitor versus a broad inhibitor of multiple kinases that could prove relevant to leukemia biology. In general, the incomplete understanding of many signal transduction inhibitors’ true mechanism of action limits our ability to identify pretreatment predictors of response. To this end, the extensive measures applied to correlate the biologic activity of FLT3 inhibitors with clinical responses are noteworthy and provide useful lessons for clinical trial design and drug development both in leukemia and other cancers.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •Of importance
Sanz MA et al., A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood, 1999. 94(9): p. 3015–3021
Cassileth PA et al., Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med, 1998. 339(23): p. 1649–1656
Rowe JM et al., A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: a trial by the Eastern Cooperative Oncology Group. Blood, 2004. 103(2): p. 479–485
Slovak ML et al., Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood, 2000. 96(13): p. 4075–4083
Nakao M et al., Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia, 1996. 10(12): p. 1911–1918
Shen WP et al., Expression of normal and mutant ras proteins in human acute leukemia. Oncogene, 1987. 1(2): p. 157–165
Yamamoto Y et al., Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood, 2001. 97(8): p. 2434–2439
Guzman ML et al., Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood, 2001. 98(8): p. 2301–2307
Guzman ML et al., Preferential induction of apoptosis for primary human leukemic stem cells. PNAS, 2002. 99(25): p. 16220–16225
Kubota Y et al., Constitutive activation of PI3K is involved in the spontaneous proliferation of primary acute myeloid leukemia cells: direct evidence of PI3K activation. Leukemia, 2004. 18(8): p. 1438–1440
Zhao S et al., Inhibition of phosphatidylinositol 3-kinase dephosphorylates BAD and promotes apoptosis in myeloid leukemias. Leukemia, 2004. 18(2): p. 267–275
Xu Q et al., Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood, 2003. 102(3): p. 972–980
Grandage VL et al., PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-kappaB, Mapkinase and p53 pathways. Leukemia, 2005. 19(4): p. 586–594
Xu Q, JE Thompson, M Carroll, mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood, 2005. 106(13): p. 4261–4268
Milella M et al., Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest, 2001. 108(6): p. 851–859
Gilliland DG, Jordan CT, Felix CA: The molecular basis of leukemia. Hematology (Am Soc Hematol Educ Program) 2004:80–97
Druker BJ, Imatinib: paradigm or anomaly? Cell Cycle, 2004. 3(7): p. 833–835
Druker BJ, NB Lydon, Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest, 2000. 105(1): p. 3–7
Gilliland DG, JD Griffin, The roles of FLT3 in hematopoiesis and leukemia. Blood, 2002. 100(5): p. 1532–1542
Levis M, D Small, Small molecule FLT3 tyrosine kinase inhibitors. Curr Pharm Des, 2004. 10(11): p. 1183–1193
Stone RM et al., Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood, 2005. 105(1): p. 54–60
DeAngelo DJ et al., Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood, 2006. 108(12): p. 3674–3681
Smith BD et al., Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood, 2004. 103(10): p. 3669–3676
Fiedler W, et al., A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood, 2005. 105(3): p. 986–993
Karbowniczek M, EP Henske, The role of tuberin in cellular differentiation: are B-Raf and MAPK involved? Ann N Y Acad Sci 2005. 1059: p. 168–173
Piloto O, et al.: Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood 2006
Gorre ME et al., Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science, 2001. 293(5531): p. 876–880
Heidel F et al., Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood, 2006. 107(1): p. 293–300
Levis M et al., Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood, 2006. 108(10): p. 3477–3483
Stone RM et al., Phase IB study of PKC412, an oral FLT3 kinase inhibitor, in sequential and simultaneous combinations with daunorubicin and cytarabine (da) induction and high-dose cytarabine consolidation in newly diagnosed adult patients (pts) with acute myeloid leukemia (AML) under age 61. Blood (ASH Annual Meeting Abstracts), 2006. 108(11): p. 157
DeAngelo DJ, et al.: Phase 1/2 study of tandutinib (MLN518) plus standard induction chemotherapy in newly diagnosed acute myelogenous leukemia (AML). Blood (ASH Annual Meeting Abstracts) 2006, 108(11):158
Levis M et al., A randomized, open-label study of lestaurtinib (CEP-701), an oral FLT3 inhibitor, administered in sequence with chemotherapy in patients with relapsed AML harboring FLT3 activating mutations: clinical response correlates with successful FLT3 inhibition. Blood, 2005. 106 (11): p. 121a
Karp JE et al., Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase 1 clinical-laboratory correlative trial. Blood, 2001. 97(11): p. 3361–3369
Harousseau JL et al., A phase 2 study of the oral farnesyltransferase inhibitor tipifarnib in patients with refractory or relapsed acute myeloid leukemia. Blood, 2007. 109(12): p. 5151–5156
Lancet JE et al., A phase 2 study of the farnesyltransferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood, 2007. 109(4): p. 1387–1394
Fenaux P et al., A multicenter phase 2 study of the farnesyltransferase inhibitor tipifarnib in intermediate- to high-risk myelodysplastic syndrome. Blood, 2007. 109(10): p. 4158–4163
Zhu K et al., Farnesyltransferase inhibitor R115777 (Zarnestra, Tipifarnib) synergizes with paclitaxel to induce apoptosis and mitotic arrest and to inhibit tumor growth of multiple myeloma cells. Blood, 2005. 105(12): p. 4759–4766
Liu G et al., Enhancement of the antitumor activity of tamoxifen and anastrozole by the farnesyltransferase inhibitor lonafarnib (SCH66336). Anticancer Drugs, 2007. 18(8): p. 923–931
Karp JE, et al.: Active oral regimen for elderly adults with newly diagnosed acute myelogenous leukemia (AML): phase I trial of oral tipifarnib (T) combined with oral etoposide (E) for adults ≥age 70 who are not candidates for traditional cytotoxic chemotherapy (TCC). ASH Annual Meeting Abstracts 2006, 108(11):426
Recher C et al., Antileukemic activity of rapamycin in acute myeloid leukemia. Blood, 2005. 105(6): p. 2527–2534
Yee KW et al., Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res, 2006. 12(17): p. 5165–5173
Yee KWL et al., A Phase II Study of Temsirolimus (CCI-779) in Patients with Advanced Leukemias. Blood (ASH Annual Meeting Abstracts), 2004. 104(11): p. 4523a
Zeng Z, et al.: Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood 2006
Luger S, et al.: A phase I dose escalation study of the mTOR inhibitor sirolimus and MEC chemotherapy targeting signal transduction in leukemic stem cells for acute myeloid leukemia. ASH Annual Meeting Abstracts 2006, 108(11):161
Mrozek K, Bloomfield CD: Chromosome aberrations, gene mutations and expression changes, and prognosis in adult acute myeloid leukemia. Hematology (Am Soc Hematol Educ Program) 2006:169–177
Paschka P et al., Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol, 2006. 24(24): p. 3904–3911
Kindler T et al., Efficacy and safety of imatinib in adult patients with c-kit-positive acute myeloid leukemia. Blood, 2004. 103(10): p. 3644–3654
Kindler T et al., Sustained complete hematologic remission after administration of the tyrosine kinase inhibitor imatinib mesylate in a patient with refractory, secondary AML. Blood, 2003. 101(8): p. 2960–2962
Cortes J et al., Results of imatinib mesylate therapy in patients with refractory or recurrent acute myeloid leukemia, high-risk myelodysplastic syndrome, and myeloproliferative disorders. Cancer, 2003. 97(11): p. 2760–2766
Aguayo A et al., Cellular vascular endothelial growth factor is a predictor of outcome in patients with acute myeloid leukemia. Blood, 1999. 94(11): p. 3717–3721
Hussong JW, GM Rodgers, PJ Shami, Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood, 2000. 95(1): p. 309–313
Roboz GJ et al., Phase 1 study of PTK787/ZK 222584, a small molecule tyrosine kinase receptor inhibitor, for the treatment of acute myeloid leukemia and myelodysplastic syndrome. Leukemia, 2006. 20(6): p. 952–957
Karp JE et al., Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: therapy with sequential 1-beta-d-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res, 2004. 10(11): p. 3577–3585
Acknowledgments
AEP is a Special Fellow in Clinical Research of the Leukemia and Lymphoma Society, a fellow of the University of Pennsylvania Institute for Translational Medicine and Therapeutics, and a sponsored researcher of the Douglass Kroll Leukemia Research Foundation. MC is a Scholar of the Leukemia and Lymphoma Society and supported by National Cancer Institute R01 CA 100885.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perl, A.E., Carroll, M. Exploiting Signal Transduction Pathways in Acute Myelogenous Leukemia. Curr. Treat. Options in Oncol. 8, 265–276 (2007). https://doi.org/10.1007/s11864-007-0043-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-007-0043-z