Abstract
Introduction
Neurological impairment is a big concern in the development of patients with congenital heart defects (CHD). A number of neuromarkers have been studied in search of a diagnostic or prognostic marker for brain injury during the vulnerable perioperative period. Our aim was to assess two novel neuromarkers, myelin basic protein (MBP) and protein Tau (pTau), as diagnostic markers for brain injury in perioperative period in children with CHD.
Methods
Forty patients were enrolled and dichotomized based on peripheric oxygen saturation in cyanotic and non-cyanotic group. Blood samples were collected preoperative, after the induction of anesthesia, and in postoperative day 1. Neuromarker concentrations were measured using commercially available ELISA kits.
Results
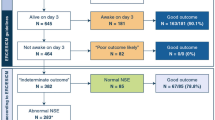
Neuromarkers’ values were increased postoperative, with statistical significance reached only in non-cyanotic group (p < 0.0001). A significant positive correlation was observed between preoperatory MBP and albumin level, hemoglobin level, height, and weight of patients. Association with cerebral saturations were analyzed by a coefficient defined as ≥ 20% reduction in cerebral saturation measured by near-infrared spectroscopy during perioperative period. An acceptable predicting model was observed with pTau in cyanotic group (AUC = 0.7).
Conclusion
We evaluated MBP and pTau as potential biomarkers of brain injury in children with CHD undergoing cardiac surgery. Elevated postoperative pTau and MBP concentrations were observed in both groups. Elevated pTau values were associated with perioperative hypoxemia.







Similar content being viewed by others
References
Go AS, Mozaffarian D, Roger VL et al (2014) Heart disease and stroke statistics – 2014 update: a report from the American heart association. Circulation 129(3):e28–e292
Pironkova RP, Giamelli J, Seiden H et al (2017) Brain injury with systemic inflammation in newborns with congenital heart disease undergoing heart surgery. Exp Ther Med 14(1):228–238
Oster ME, Lee KA, Honein MA et al (2013) Temporal trends in survival among infants with critical congenital heart defects. Pediatrics 131(5):e1502–e1508
Gaynor JW, Stopp C, Wypij D et al (2015) Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 135(5):816–825
Marino BS, Lipkin PH, Newburger JW et al (2012) American heart association congenital heart defects committee, council on cardiovascular disease in the young, council on cardiovascular nursing, and stroke council, neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American heart association. Circulation 126(9):1143–1172
Dimitropoulos A, McQuillen PS, Sethi V et al (2013) Brain injury and development in newborns with critical congenital heart disease. Neurology 81(3):241–248
Graham EM, Martin RH, Atz AM et al (2019) Association of intraoperative circulating-brain injury biomarker and neurodevelopmental outcomes at 1 year among neonates who have undergone cardiac surgery. J Thorac Cardiovasc Surg 157(5):1996–2002
Chiperi LE, Tecar C, Toganel R (2023) Neuromarkers which can predict neurodevelopmental impairment among children with congenital heart defects after cardiac surgery: a systematic literature review. Dev Neurorehabil 26(3):206–215
Trakas E, Domnina Y, Panigrahy A et al (2017) Serum neuronal biomarkers in neonates with congenital heart disease undergoing cardiac surgery. Pediatr Neurol 72:56–61
Sanchez-de-Toledo J, Chrysostomou C, Munoz R et al (2014) Cerebral regional oxygen saturation and serum neuromarkers for the prediction of adverse neurologic outcome in pediatric cardiac surgery. Neurocrit Care 21(1):133–139
Schmitt B, Bauersfeld U, Schmid ER et al (1998) Serum and CSF levels of neuron-specific enolase (NSE) in cardiac surgery with cardiopulmonary bypass: a marker of brain injury? Brain Dev 20(7):536–539
Abella R, Varrica A, Satriano A et al (2015) Biochemical markers for brain injury monitoring in children with or without congenital heart diseases. CNS Neurol Disord Drug Targets 14(1):12–23
Robertson DR, Justo RN, Burke CJ et al (2014) Perioperative predictors of developmental outcome following cardiac surgery in infancy. Cardiol Young 14(4):389–395
Bar-Yosef O, Greidinger D, Iskilova M et al (2018) Neurological deficit is predicted by S100B in children after cardiac surgery. Clin Chim Acta 481:56–60
Lardner D, Davidson A, McKenzie I, Cochrane A (2004) Delayed rises in serum S100B levels and adverse neurological outcome in infants and children undergoing cardiopulmonary bypass. Paediatr Anaesth 14(6):495–500
Vergine M, Vedovelli L, Simonato M et al (2021) Perioperative glial fibrillary acidic protein is associated with long-term neurodevelopment outcome of infants with congenital heart disease. Children (Basel) 8:655
Vedovelli L, Padalino M, Suppiej A et al (2018) Cardiopulmonary-bypass glial fibrillary acidic protein correlates with neurocognitive skills. Ann Thorac Surg 106(3):792–798
Lee T, Chikkabyrappa SM, Reformina D et al (2018) Ubiquitin C-terminal hydrolase 1 and phosphorylated axonal neurofilament heavy chain in infants undergoing cardiac surgery: preliminary assessment as potential biomarkers of brain injury. World J Pediatr Congenit Heart Surg 9(4):412–418
Gessler P, Pretre R, Hohl V et al (2004) CXC-chemokine stimulation of neutrophils correlates with plasma levels of myeloperoxidase and lactoferrin and contributes to clinical outcome after pediatric cardiac surgery. Shock 22(6):513–520
Bembea MM, Rizkalla N, Freedy J et al (2015) Plasma biomarkers of brain injury as diagnostic tools and outcome predictors after extracorporeal membrane oxygenation. Crit Care Med 43(10):2202–2211
Mörtberg E, Zetterberg H, Nordmark J et al (2011) Plasma tau protein in comatose patients after cardiac arrest treated with therapeutic hypothermia. Acta Anaesthesiol Scand 55(9):1132–1138
Binder LI, Frankfurter A, Rebhun LI (1985) The distribution of tau in the mammalian central nervous system. J Cell Biol 101(4):1371–1378
Randall J, Mörtberg E, Provuncher GK et al (2013) Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation 84(3):351–356
Li R, Lee JK, Govindan RB et al (2023) Plasma biomarkers of evolving encephalopathy and brain injury in neonates with hypoxic-ischemic encephalopathy. J Pediatr 252:146–153
Liliang PC, Liang CL, Weng HC et al (2010) Tau proteins in serum predict outcome after severe traumatic brain injury. J Surg Res 160(2):302–307
Hossain I, Blennow K, Posti JP, Zetterberg H (2022) Tau as a fluid biomarker of concussion and neurodegeneration. Concussion (London, England) 7(2):CNC98
Castellani RJ, Perry G (2019) Tau biology, tauopathy, traumatic brain injury, and diagnostic challenges. J Alzheimer’s Dis 67(2):447–467
Nakamura K, Greenwood A, Binder L et al (2012) Proline isomer-specific antibodies reveal the early pathogenic tau conformation in Alzheimer’s disease. Cell 149(1):232–244
Fink EL, Berger RP, Clark RS et al (2014) Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest. Crit Care Med 42(3):664–674
Sandler SJ, Figaji AA, Adelson PD (2010) Clinical applications of biomarkers in pediatric traumatic brain injury. Child’s nervous system 26(2):205–213
Thomas DG, Palfreyman JW, Ratcliffe JG (1978) Serum-myelin-basic-protein assay in diagnosis and prognosis of patients with head injury. Lancet 1:113–115
Ghaith HS, Nawar AA, Gabra MD et al (2022) A literature review of traumatic brain injury biomarkers. Mol Neurobiol 59(7):4141–4158
Graham EM, Everett AD, Delpech JC, Northington FJ (2018) Blood biomarkers for evaluation of perinatal encephalopathy: state of the art. Curr Opin pediatr 30(2):199–203
Lv H, Wang Q, Wu S et al (2015) Neonatal hypoxic ischemic encephalopathy-related biomarkers in serum and cerebrospinal fluid. Clinica chimica acta. International J Clin Chem 450:282–297
Wang Q, Lv H, Wu S et al (2022) Effect of hypothermia on serum myelin basic protein and tumor necrosis factor-α in neonatal hypoxic-ischemic encephalopathy. Am J Perinatol 39(12):1367–1374
Schiller O, Goldshmid O, Mowassi S et al (2020) The utility of albumin level as a marker of postoperative course in infants undergoing repair of congenital heart disease. Pediatr Cardiol 41(5):939–946
Van Beek DEC, Van Der Horst ICC, De Geus AF et al (2018) Albumin, a marker for post-operative myocardial damage in cardiac surgery. J Crit Care 47:55–60
Leite HP, da Silva AVR, de Oliveira Iglesias SB, Nogueira PCK (2016) Serum albumin is an independent predictor of clinical outcomes in critically ill children. Pediatr Crit Care Med 17(2):e50–e57
Kapoor P, Narula J, Chowdhury U et al (2016) Serum albumin perturbations in cyanotics after cardiac surgery: patterns and predictions. Ann Card Anaesth 19:300
Hansen JH, Kissner L, Logoteta J et al (2019) S100B and its relation to cerebral oxygenation in neonates and infants undergoing surgery for congenital heart disease. Congenit Heart Dis 14(3):427–437
Kurth CD, Levy WJ, McCann J (2002) Near-infrared spectroscopy cerebral oxygen saturation thresholds for hypoxia-ischemia in piglets. J Cereb Blood flow Metab: Offic J Int Soc Cereb Blood Flow Metab 22(3):335–341
Dietrick B, Molloy E, Massaro AN et al (2020) Plasma and cerebrospinal fluid candidate biomarkers of neonatal encephalopathy severity and neurodevelopmental outcomes. J Pediatr 226:71–79
Massaro AN, Wu YW, Bammler TK et al (2018) Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J Pediatr 194:67–75
Acknowledgements
The authors would like to thank Mihaita George Gavra and Liliana Demian for their help in laboratory work.
Funding
This work was supported by the University of Medicine, Pharmacy, Science and Technology “George Emil Palade,” Targu Mures, Research Grant number 510/3/17.01.2022. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
L-EC: conception and design of the work; acquisition, analysis, and interpretation of data for the work; drafting the manuscript; approval of the final manuscript; accountable for all aspects of the work; obtained fundings; gathered materials for the work; collected data and processed it; responsible for the analysis and interpretation of data; review the literature; wrote the manuscript; part of laboratory work; accountable for all aspects of the work. CT: review the literature; wrote a part of the manuscript; critically revising the manuscript for important intellectual content; approval of the final manuscript; accountable for all aspects of the work. Adina Huţanu: underdone the laboratory work; critically revising the manuscript for important intellectual content; approval of the final manuscript
Corresponding author
Ethics declarations
Ethics approval
Document number 1161 form October 26, 2020—Ethics committee of George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Targu Mures, Romania.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chiperi, L.E., Tecar, C. & Huţanu, A. Serum tau protein and myelin basic protein in pediatric patients with congenital heart defects undergoing cardiac surgery: preliminary assessment as novel neuromarkers of brain injury. Ir J Med Sci 193, 1229–1237 (2024). https://doi.org/10.1007/s11845-023-03582-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-023-03582-5