Abstract
Background
Diabetic foot ulcer (DFU) carries high rates of major amputation and mortality.
Aims
The goals of this study were to identify expression of circulating lncRNA DLEU1 and miR-96-5p in patients with diabetic foot ulcer (DFU) and to explore the function of lncRNA DLEU1/miR-96-5p axis in DFU.
Methods
Matched patients with DFU and healthy individuals were randomly selected. Serum samples from all subjects were used for circulating lncRNA DLEU1 and miR-96-5p assessment by RT-qPCR. Receiver operating characteristic (ROC) curve was plotted to assess the discriminative capacity of lncRNA DLEU1 and miR-96-5p in identifying DFU. Cell proliferation was detected by CCK-8 assay. Cell apoptosis was assayed by Annexin V-FITC/PI staining method. Bioinformatics, luciferase reporter activity assay, and in vitro cell experiments were used to explore the relationship between lncRNA DLEU1 and miR-96-5p.
Results
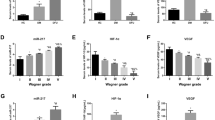
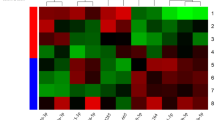
LncRNA DLEU1 and miR-96-5p were significantly up- and downregulated in patients with DFU, respectively, compared with controls. After ROC assessment, lncRNA DLEU1 and miR-96-5p were found to discriminate DFU from miR-96-5p. Furthermore, lncRNA DLEU1 inhibited human umbilical vein endothelial cells (HUVECs) cell proliferation and increased HUVECs apoptosis and oxidative stress through sponging miR-96-5p.
Conclusion
Our findings suggest lncRNA DLEU1 and miR-96-5p as circulating biomarkers for DFU. Also, we provide the clue for the pathogenic significance of lncRNA DLEU1/miR-96-5p in DFU, as well as insights for new potential targets.





Similar content being viewed by others
References
Reardon R, Simring D, Kim B et al (2020) The diabetic foot ulcer. Australian J Gen Pract 49:250–255. https://doi.org/10.31128/ajgp-11-19-5161
Lovic D, Piperidou A, Zografou I et al (2020) The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol 18:104–109. https://doi.org/10.2174/1570161117666190405165911
McDermott K, Fang M, Boulton AJM et al (2023) Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care 46:209–221. https://doi.org/10.2337/dci22-0043
Sorber R, Abularrage CJ (2021) Diabetic foot ulcers: epidemiology and the role of multidisciplinary care teams. Semin Vasc Surg 34:47–53. https://doi.org/10.1053/j.semvascsurg.2021.02.006
Chi T, Lin J, Wang M et al (2021) Non-Coding RNA as Biomarkers for Type 2 Diabetes Development and Clinical Management. Front Endocrinol 12:630032. https://doi.org/10.3389/fendo.2021.630032
Li Y (2021) Modern epigenetics methods in biological research. Methods (San Diego, Calif) 187:104–113. https://doi.org/10.1016/j.ymeth.2020.06.022
Faruq O, Vecchione A (2015) microRNA: diagnostic perspective. Front Med 2:51. https://doi.org/10.3389/fmed.2015.00051
Xu YX, Pu SD, Li X et al (2022) Exosomal ncRNAs: Novel therapeutic target and biomarker for diabetic complications. Pharmacol Res 178:106135. https://doi.org/10.1016/j.phrs.2022.106135
Saw PE, Xu X, Chen J, Song EW (2021) Non-coding RNAs: the new central dogma of cancer biology. Science China Life sciences 64:22–50. https://doi.org/10.1007/s11427-020-1700-9
Zhang P, Wu W, Chen Q, Chen M (2019) Non-coding RNAs and their integrated networks. Journal of integrative bioinformatics 16. https://doi.org/10.1515/jib-2019-0027
Patel S, Srivastava S, Singh MR, Singh D (2019) Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 112:108615. https://doi.org/10.1016/j.biopha.2019.108615
Burgess JL, Wyant WA, Abdo Abujamra B et al (2021) Diabetic wound-healing science. Medicina (Kaunas, Lithuania) 57. https://doi.org/10.3390/medicina57101072
Song C, Zhang J, Zhao Z et al (2020) DLEU1: a functional long noncoding RNA in tumorigenesis. Curr Pharm Des 26:1742–1748. https://doi.org/10.2174/1381612826666200122145305
Yan C, Chen J, Yang X et al (2021) Emerging roles of long non-coding RNAs in Diabetic foot ulcers. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 14:2549–2560. https://doi.org/10.2147/dmso.S310566
Yu P, Guo J, Li J et al (2019) Co-expression network analysis revealing the key lncRNAs in diabetic foot ulcers. Arch Med Sci: AMS 15:1123–1132. https://doi.org/10.5114/aoms.2019.84699
Xu S, Weng X, Wang Y et al (2019) Screening and preliminary validation of T lymphocyte immunoregulation-associated long non-coding RNAs in diabetic foot ulcers. Mol Med Rep 19:2368–2376. https://doi.org/10.3892/mmr.2019.9877
Tu L, Huang Q, Hu Y, Liu D (2020) Detection and analysis of angiogenesis pathway-associated lncRNA expression profiles in human skin fibroblasts under high-glucose conditions. Mol Med Rep 22:2283–2290. https://doi.org/10.3892/mmr.2020.11333
Fan CX, Huang ZQ, Chen BB et al (2019) Comprehensive analysis of key lncRNAs in ischemic stroke. Mathematic Biosci Eng: MBE 17:1318–1328. https://doi.org/10.3934/mbe.2020066
Ren S, Xiong H, Chen J et al (2021) The whole profiling and competing endogenous RNA network analyses of noncoding RNAs in adipose-derived stem cells from diabetic, old, and young patients. Stem Cell Res Ther 12:313. https://doi.org/10.1186/s13287-021-02388-5
Zhu L, Zhong Q, Yang T, Xiao X (2019) Improved therapeutic effects on diabetic foot by human mesenchymal stem cells expressing MALAT1 as a sponge for microRNA-205–5p. Aging 11:12236–12245. https://doi.org/10.18632/aging.102562
Li B, Luan S, Chen J et al (2020) The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol Therapy Nucleic Acids 19:814–826. https://doi.org/10.1016/j.omtn.2019.11.034
Wu X, Yin S, Yan L et al (2022) lncRNA DLEU1 Modulates proliferation, inflammation, and extracellular matrix degradation of chondrocytes through regulating miR-671-5p. J Immunol Res 2022:1816217. https://doi.org/10.1155/2022/1816217
Madhyastha R, Madhyastha H, Nakajima Y et al (2012) MicroRNA signature in diabetic wound healing: promotive role of miR-21 in fibroblast migration. Int Wound J 9:355–361. https://doi.org/10.1111/j.1742-481X.2011.00890.x
Zolfaghari N, Soheili ZS, Samiei S et al (2023) microRNA-96 targets the INS/AKT/GLUT4 signaling axis: association with and effect on diabetic retinopathy. Heliyon 9:e15539. https://doi.org/10.1016/j.heliyon.2023.e15539
Yu X, Liu Z, Fang J, Qi H (2021) miR-96–5p: a potential diagnostic marker for gestational diabetes mellitus. Medicine 100:e25808. https://doi.org/10.1097/md.0000000000025808
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, M., Gu, Y. LncRNA DLEU1 promotes angiogenesis in diabetic foot ulcer wound healing by regulating miR-96-5p. Ir J Med Sci 193, 241–247 (2024). https://doi.org/10.1007/s11845-023-03471-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-023-03471-x