Abstract
Background
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder that affects the processing of carbohydrates, proteins, and lipids. In T2DM, metabolic dysregulation occurs through various pathways caused by increased levels of many adipokines and inflammatory chemokines. Impaired insulin-glucose metabolism occurs in tissues. The proteolytic enzyme matriptase is thought to be closely related to glucose metabolism due to its glycolization sites.
Aim
Our study aimed to evaluate the correlation between matriptase, a proteolytic enzyme, and metabolic parameters in individuals recently diagnosed with T2DM. We also sought to investigate the potential involvement of matriptase in the development of diabetes.
Methods
We measured all participants' metabolic laboratory parameters, including basic biochemical tests, hemograms, high-sensitivity C-reactive protein (hsCRP), and matriptase levels.
Results
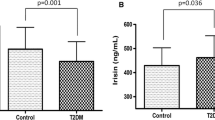
Our results showed a significant increase in circulating matriptase levels in individuals with T2DM compared to the control group. Furthermore, individuals with metabolic syndrome had significantly higher matriptase levels than those without in the T2DM and control groups. We also observed that T2DM patients had elevated levels of Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), hsCRP, and matriptase, which displayed a positive correlation.
Conclusion
Our study is the first to report elevated levels of matriptase in individuals with newly diagnosed T2DM and/or metabolic syndrome. Additionally, we found a significant positive correlation between matriptase levels and metabolic and inflammatory parameters, indicating a potential role for matriptase in the pathogenesis of T2DM and glucose metabolism. Further research on matriptase could lead to its recognition as a novel target for investigation.



Similar content being viewed by others
Data availability
Data and materials are reachable from hospital automation information systems.
References
DeFronzo RA, Ferrannini E, Groop L et al (2015) Type 2 diabetes mellitus. Nat Rev Dis Primers 1:15019. https://doi.org/10.1038/nrdp.2015.19
Poznyak A, Grechko AV, Poggio P et al (2020) The diabetes mellitus–atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci 21(5):1835. https://doi.org/10.3390/ijms21051835
Nicholson T, Church C, Baker DJ et al (2018) The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J Inflamm (Lond) 15:9. https://doi.org/10.1186/s12950-018-0185-8
Al-Hamodi Z, Al-Habori M, Al-Meeri A et al (2014) Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetol Metab Syndr 6(1):99. https://doi.org/10.1186/1758-5996-6-99
Antalis TM, Bugge TH, Wu Q (2011) Membrane-anchored serine proteases in health and disease. Prog Mol Biol Translation Sci 99:1–50. https://doi.org/10.1016/B978-0-12-385504-6.00001-4
Anand D, Hummler E, Rickman OJ (2022) ENaC activation by proteases. Acta Physiologica 235(1):e13811. https://doi.org/10.1111/apha.13811
Oberst MD, Singh B, Ozdemirli M et al (2003) Characterization of matriptase expression in normal human tissues. J Histochem Cytochem 51(8):1017–1025. https://doi.org/10.1177/002215540305100805
Ihara S, Miyoshi E, Nakahara S et al (2004) Addition of β1-6 GlcNAc branching to the oligosaccharide attached to Asn 772 in the serine protease domain of matriptase plays a pivotal role in its stability and resistance against trypsin. Glycobiology 14(2):139–146. https://doi.org/10.1093/glycob/cwh013
Netzel-Arnett S, Hooper JD, Szabo R et al (2003) Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev 22(2–3):237–258. https://doi.org/10.1023/a:1023003616848
Galicia-Garcia U, Benito-Vicente A, Jebari S et al (2020) Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci 21(17):6275. https://doi.org/10.3390/ijms21176275
American Diabetes Association Professional Practice Committee (2022) 2 Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care 45(Supplement_1):S17–S38. https://doi.org/10.2337/dc22-S002
Grundy SM, Cleeman JI, Daniels SR et al (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112(17):2735–2752. https://doi.org/10.1161/CIRCULATIONAHA.105.169404
Kahn BB (1998) Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell 92(5):593–596. https://doi.org/10.1016/s0092-8674(00)81125-3
Cheng HL, Hancock DP, Rooney KB et al (2014) A candidate gene approach for identifying differential iron responses in young overweight women to an energy-restricted haem iron-rich diet. Eur J Clin Nutr 68(11):1250–1252. https://doi.org/10.1038/ejcn.2014.82
Ahmed S, Jin X, Yagi M et al (2006) Identification of membrane-bound serine proteinase matriptase as processing enzyme of insulin-like growth factor binding protein-related protein-1 (IGFBP-rP1/angiomodulin/mac25). The FEBS J 273(3):615–627. https://doi.org/10.1111/j.1742-4658.2005.05094.x
Silvestri L, Pagani A, Nai A et al (2008) The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab 8(6):502–511. https://doi.org/10.1016/j.cmet.2008.09.012
Nai A, Pagani A, Silvestri L et al (2011) TMPRSS6 rs855791 modulates hepcidin transcription in vitro and serum hepcidin levels in normal individuals. Blood 118(16):4459–4462. https://doi.org/10.1182/blood-2011-06-364034
Sam AH, Busbridge M, Amin A et al (2013) Hepcidin levels in diabetes mellitus and polycystic ovary syndrome. Diabetic Med 30(12):1495-1499. https://doi.org/10.1111/dme.12262
Uhland K (2006) Matriptase and its putative role in cancer. Cell Mol Life Sci CMLS 63(24):2968–2978. https://doi.org/10.1007/s00018-006-6298-x
Rawlings ND, Salvesen G (2013) Handbook of proteolytic enzymes (Vol. 3). Academic Press
Terry KL, Sluss PM, Skates SJ et al (2004) Blood and urine markers for ovarian cancer: a comprehensive review. Dis Markers 20(2):53–70. https://doi.org/10.1155/2004/241982
Oberst M, Anders J, Xie B et al (2001) Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo. Am J Pathol 158(4):1301–1311. https://doi.org/10.1016/S0002-9440(10)64081-3
Shigetomi H, Onogi A, Kajiwara H et al (2010) Anti-inflammatory actions of serine protease inhibitors containing the Kunitz domain. Inflam Res 59(9):679–687. https://doi.org/10.1007/s00011-010-0205-5
Redondo‐Calvo FJ, Padín JF, Muñoz‐Rodríguez JR et al (2022) Aprotinin treatment against SARS‐CoV‐2: A randomized phase III study to evaluate the safety and efficacy of a pan‐protease inhibitor for moderate COVID‐19. Eur J Clin Investigation e13776. https://doi.org/10.1111/eci.13776
Funding
This study has not been funded by any organizations.
Author information
Authors and Affiliations
Contributions
Conceptualization: [Ismail Demir], [Ismail Yilmaz], [Oktay Bilgir]; Methodology: [Ismail Demir], [Ismail Yilmaz], [Oktay Bilgir]; Investigation: [Ismail Demir], [Ismail Yilmaz], [Bulent Calik]; Writing-Original Draft: [Ismail Demir], [Ismail Yilmaz], [Ersan Horoz]; Writing-Review&Editing: [Ismail Yilmaz], [Oktay Bilgir], [Bulent Calik], [Ersan Horoz]. All authors have read and approved the final version.
Corresponding author
Ethics declarations
Ethical approval
This study was reviewed and approved by the institutional review board for exemption.
Consent to participate
Informed consent was obtained from all participants included in the study.
Conflict of interest
The authors declare no competing interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Demir, I., Yilmaz, I., Horoz, E. et al. Matriptase as a potential biomarker and therapeutic target in newly diagnosed type 2 diabetes mellitus. Ir J Med Sci 193, 223–230 (2024). https://doi.org/10.1007/s11845-023-03441-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-023-03441-3