Abstract
Background
While the prevalence of type 2 diabetes (T2D) is growing worldwide, dietary intake plays a remarkable role in the management of disease complications. Evidence suggests that beetroot has health-promoting potentials, including anti-inflammatory, antioxidant, and antidiabetic properties. Therefore, the present clinical trial aimed to investigate the effects of concentrated beetroot juice (BJ) supplementation on anthropometric measures, glycemic control, blood pressure (BP), and lipid profile in T2D patients.
Methods
In the simply randomized, parallel-group, controlled, and open-label trial, forty-six patients with T2D were randomly allocated to either the intervention group (BJ group), who consumed 24 ml concentrated BJ daily for 12 weeks, or the control group without any intervention. Anthropometric measurements, physical activity, dietary intakes, glycemic measures, lipid profile, and blood pressure were assessed at the baseline and the end of the study.
Results
Plasma nitric oxide (NO) in the intervention group had a higher nonsignificant increase after 12 weeks compared with the control group (8.57 ± 23.93 vs. 2.31 ± 15.98, P = 0.128). Compared with the baseline, significant reductions in plasma insulin (14.55 ± 7.85 vs. 10.62 ± 6.96, P = 0.014) and homeostasis model assessment of β-cell function (HOMA-B) (3.96 ± 0.83 vs. 3.63 ± 0.75, P = 0.038), as well as a marginally significant reduction in high-density lipoprotein cholesterol (HDL-C) (70.81 ± 11.24 vs. 65.44 ± 6.46, P = 0.058) were observed in the control group after 12 weeks. Diastolic blood pressure (DBP) was significantly reduced in the BJ group compared with the baseline (74.73 ± 16.78 vs. 72.36 ± 16.09, P = 0.046). After adjusting for baseline values, no significant effect on the levels of fasting plasma glucose (FPG), insulin, hemoglobin A1c (HgA1c), HOMA-β, homeostatic model assessment for insulin resistance (HOMA-IR), total cholesterol (TC), low-density lipoprotein (LDL), HDL, triglycerides (TG), and blood pressure (BP) was observed.
Conclusions
Our study showed that daily consumption of 24 ml concentrated BJ did not affect the levels of glycemic measures, blood pressure, and lipid profile. More studies are necessary to confirm these findings.
Trial Registration
This present clinical trial has been registered in the Iranian Registry of Clinical Trials with registration number IRCT20150815023617N5.
Similar content being viewed by others
Background
Type 2 diabetes (T2D) also known as insulin resistance hyperglycemia leads to micro-and macro-vascular complications [1, 2]. According to World Health Organization (WHO) report in 2016, approximately 422 million people have been diagnosed with T2D around the world, and about 1.6 million deaths are annually attributed to T2D [1]. Several factors, including dietary intake, have a remarkable role in the development of T2D [3]. Beetroot (Beta vulgaris L.) is known as a potent antioxidant plant because of its high content of biologically active phytochemicals including polyphenols, saponins, flavonoids, betalains (such as betacyanins and betaxanthins), and inorganic nitrate (NO3) (within a range of 388 ± 19.9 to 3968 ± 252 mg/L among commercial beetroot juice) [4,5,6]. Evidence demonstrates that beetroot, along with its functional properties, has health-promoting potential, including anti-inflammatory, antioxidant, anti-carcinogenic, antidiabetic, hepato-protective, and wound healing properties [7,8,9]. The implications of beetroot and its by-products have been noticed on insulin and glucose responses within the glycemic homeostatic context through the activation of AMPK signaling, reducing carbohydrate digestion, and suppressing the postprandial glucose response in several studies [7, 10, 11]. Furthermore, beetroot consumption could impact both systolic and diastolic blood pressures, as well as vascular and endothelial function. It is noticeable that some studies have indicated the effect of NO on the lipid profile because of its complementary role in the occurrence of glycemic abnormalities [4, 12,13,14].
Whether the beneficial effects of beetroot juice (BJ) are due to the influences of nitrate or other bioactive compounds remains controversial [4, 5, and 31]. Based on the literature, beneficial properties of NO3/NO2 on glucose and insulin homeostasis have also been reported in experimental and animal studies [15, 16], though its positive effects in human T2D patients have yet to be proven [17]. To the best of our knowledge, most of the previous studies investigated the positive effects of consuming nitrate-rich BJ compared with NO3-depleted BJ [18,19,20]. Two human studies conducted by Gilchrist et al. [18] and Bahadoran et al. [17] showed no effects of dietary nitrate on insulin resistance and metabolic indices in T2DM patients. Therefore, the present clinical trial study aimed to investigate the effects of total bioactive contents of concentrated BJ supplementation on glycemic control, systolic and diastolic blood pressures, and lipid profile in T2D patients.
Materials and methods
Study population
Participants in the present trial were 35- to 70-year-old T2D patients with body mass index (BMI) higher than 18.5 kg/m2 who had attended Shahid Ghasem Clinic, Sari, Iran for medical care. All participants were diagnosed with diabetes during the last 1–10 years according to Standards of Medical Care in Diabetes Guidelines (American Diabetes Association 2019) and were treated with oral hypoglycemic agents. Patients were not included in the trial if they were suffering from inflammatory diseases, hepatic, and renal disorders or had a history of micro-and macro-vascular diseases (such as a history of angioplasty and foot vascular surgery) or COVID-19. Patients with an HgA1c ≥ 11 or those who consume multivitamin and mineral/herbal supplementary medications, glucocorticoids, nonsteroidal anti-inflammatory drugs, and nitrate-containing drugs such as nitroglycerin, isosorbide, nitropress, etc., during last 3 months and individuals who had been on a weight loss diet within the last 6 months were also excluded from the study. We also excluded any patients who were or became pregnant and/or breastfeeding during the study period. The clinical trial was carried out between October 2018 and December 2019 and was conducted in accordance with the Declaration of Helsinki and the Consolidated Standards of Reporting Trials (CONSORT) statement for randomized trials [21]. At the baseline, the study goals were described to all participants, and written informed consent was completed by each participant. Patients were asked to keep their routine dietary intake, physical activity habits, and usual medicine intake during the study; otherwise, they were excluded from the study. The study protocol was approved by the Ethics Committee of the National Nutrition and Food Technology Research Institute of Iran (IR.SBMU.nnftri.Rec.1399.035). This clinical trial was registered at the Iranian Registry of Clinical Trials (IRCT) with the following identification: IRCT20150815023617N5.
Study design and measurements
The present clinical trial was a simply randomized, parallel-group, controlled, open-label study. Block randomization method using a computer algorithm, written in SAS® (Cary, NC), was used to randomly allocate participants to either the intervention group (BJ group) or the control group after matching for sex and age. The BJ group consumed 24 ml concentrated BJ each day between main meals at the same time in two doses for 12 weeks, and the control group received no intervention and was asked not to change their routine lifestyle for the same time. Concentrated BJ was prepared from fresh beetroot (Beta vulgaris var. Esculenta, Chenopodiaceae family) commercially (Takdaneh, Inc., Marand, Iran) without any additives. They administered 24 ml of concentrated BJ equivalent to 240 ml beetroot juice which contains 180 mg nitrate and 0.1 mg nitrite. Based on previous studies, daily consumption of 25 ml concentrated BJ does not increase the blood glucose levels in T2D patients [22]. During this trial, all the patients were advised to go on a low-nitrate diet which limited their intake of nitrate-rich foods such as cabbage, beetroot, rocket, spinach, mint, tarragon, radish, processed meat, etc. to allow the test results to be as clear as possible. This way, the patients received a minimum of 180 mg of nitrate daily, which is slightly lower than the accepted daily intake value (3.7 mg/kg for NO3 and 0.06 mg/kg for NO2) [23, 24].
Sample size
The study sample size was calculated using the sample size formula for randomized clinical trials. According to previous studies [25], assuming the statistical power of 0.80 and a significance of 0.05, in order to detect a significant difference of 28.6 mg/dl (SD:29 mg/dl) in the glucose level, a minimum of 19 subjects was determined for each study group. Due to the 20% potential drop-out of the patients, a total of 8 extra patients were added to the calculated sample size. Finally, 46 T2DM patients (23 participants in each study group) enrolled in the study.
Follow-up assessments and compliance
The study participants were contacted by phone every 15 days to verify if they have any problems with the consumption of concentrated BJ and inquired regarding possible side effects. A bottle of concentrated BJ, adequate for 30 days, was given to the BJ group at baseline. The second and the third bottles were given at the follow-up visits within 4 weeks. The concentrated BJ was stored at 0 to 1 °C before distribution, and the BJ group was asked to keep it at refrigerator temperature (less than 5 °C). After 12 weeks, the participants were asked to return the unused bottles. The criteria for patient compliance were a minimum of 90%; thus, any patients who did not meet this standard were regarded as noncompliant and removed from the trial. The participants’ compliance was evaluated based on the number of returned bottles. Plasma nitrate and nitrite were measured at the baseline and after 12 weeks of intervention.
Anthropometric, dietary, and physical activity assessment
A general questionnaire, regarding demographic information, present illnesses, drug history, duration of diabetes, and prescribed medications, was filled out at baseline. Height, weight, as well as waist and hip circumferences, were measured by an expert nutritionist at the baseline and the end of the study. Weight was measured using a calibrated Seca balance scale (Seca, Hamburg, Germany), with a precision of 0.1 kg, while subjects were wearing light clothing and no shoes. Height was measured using a stadiometer (Seca, Hamburg, Germany), while subjects were standing without shoes, with their shoulders in a normal position. Waist and hip circumferences were measured using flexible tape. Waist circumference (WC) was measured at the midpoint between the iliac crest and the lowest rib. Hip circumference (HC) was measured around the widest portion of the buttocks. BMI and waist-to-hip ratio (WHR) were calculated according to WHO recommendations [25].
Dietary intake during the study period was measured using 24-h dietary recalls over 3 days (one weekend and two weekdays) at the beginning and end of the study. The first recall was recorded by a professional dietitian during an in-person meeting, and the other two were recorded over the phone. The analysis was done using the Nutritionist IV software (N Squared Computing, San Bruno, CA) modified for Iranian foods. Physical activity was also evaluated at the baseline and the end of the study using a validated semi-quantitative questionnaire based on metabolic equivalent (MET)-min/week values [25]. Systolic and diastolic blood pressure was measured twice in 10-min intervals at baseline and at the end of the study for each patient. The mean values of blood pressure (BP) were then used in the study as analytic values [23].
Measurement of biochemical variables
Following overnight fasting (12–14 h), 10 ml peripheral blood samples were collected from each subject at baseline and the end of the trial. All blood samples were divided into two parts; 1 mL was used for the determination of hemoglobin HgA1c (HbA1c), and the remaining sample was immediately centrifuged (3500 rpm: 10 min at room temperature) and the separated plasma was stowed at − 80 °C for subsequent biochemical variables’ analysis. All biochemical variables were measured in the same laboratory by using standard laboratory methods. Human nitric oxide (NO) was measured using a colorimetric assay (ZellBio GmbH, Ulm, Germany). Fasting plasma glucose (FPG) was measured by using GOD/POD method (Pars Azmoon) [25]. High-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and triglycerides (TG) concentrations were measured by the photometric enzymatic method using Pars Azmoon Kit (Pars Azmoon). LDL-cholesterol concentration, calculated using the Friedwald formula (LDL = TC − HDL − (TAG/5). Fasting insulin levels were assessed by Monobind Elisa kit (Monobind) [26]. The HbA1c was measured on the whole blood sample by direct enzymatic HbA1c assay (Diazyme Laboratories, Inc., CA, USA) [25]. Level of changes in pancreatic B-cell function (HOMA-B) and homeostatic model assessment for insulin resistance (HOMA-IR) were calculated by formulas, respectively, HOMA-B = [20 × fasting insulin (μIU/ml)/glucose(mmol/l) − 3.5] and HOMA-IR = (fasting glucose (mmol/l) × fasting insulin(μIU/ml))/22.5 [27].
Concentrated beetroot juice characteristics and analytical assays
Concentrated BJ prepared by Takdaneh, Inc., Iran, without any additives (contains 180 mg nitrate and 0.1 mg nitrite per 24 ml). Total phenolic content (TPC) was measured by the Folin–Ciocalteau reagent, using gallic acid as a standard. The absorbance was measured at 765 nm using the colorimetric assay [28]. Total flavonoid content was measured by the aluminum chloride and potassium acetate. The absorbance of the reaction mixture was subsequently measured at 415 nm colorimetric assay using quercetin (10 mg/100 ml) as standard [28]. Concentrated BJ was diluted at 1:10 (v:v) to measure its total antioxidant capacity (TAC), based on the inhibition percent of DPPH. The working solution (6 × 10 − 5 M) was prepared, and then the absorbance at 515 nm was recorded using a spectrophotometer. A control with no added extract was also analyzed [29]. Nitrate and nitrite content was measured by the colorimetric assay. Nitrate content was measured according to the cadmium reduction method [30]. Sugar content was measured by the HPLC method. All the analytic data was measured during the start, middle, and end of the study. The quality of beetroot juice was assessed at weeks 0, 6, and 12 of the study by the Food and Drug Quality Control Lab, Mazandaran University of Medical Sciences, Sari, Iran. In order to assure the utmost accuracy, 3 different containers were analyzed each time. The concentrated BJ composition is summarized in Table 1.
Statistical analysis
In this study, the normality assumption for variables was assessed using the Kolmogorov–Smirnov statistic. Natural logarithm-transformed (ln) was performed to normalize the distribution of parameters with skewed distribution (cholesterol, vitamin E, vitamin C, alpha-carotene, beta-carotene, and HOMA-B). Categorical variables were reported as frequency. Continuous data were expressed in terms of mean ± standard deviation. The Student’s t-test was performed to examine the differences in baseline and endpoint characteristics of the subjects between the two groups. A paired t-test was used for within-group comparisons. Analysis of covariance (ANCOVA) was used to determine any differences at the end of the study by adjusting for baseline values and other confounding factors. A p-value less than 0.05 was considered statistically significant, based on two-sided tests. Statistical analyses were performed by the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) for Windows version 24.0.
Results
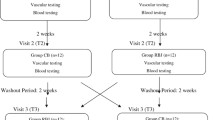
Of 200 assessed patients, forty-six subjects who met inclusion criteria were selected and enrolled in the trial. Finally, 38 individuals (19 subjects in the BJ group and 19 subjects in the control group) completed the 12-week trial. Three women and one man in the BJ group did not complete the study because of different reasons, including having a car accident, changing living areas, COVID-19 infection, and death. Two women and two men in the control group withdrew from the study because of insulin use for blood glucose control, COVID-19 infection, and not attending the last screening session because of the COVID-19 outbreak. No major side effect was reported following consuming concentrated BJ in the subjects who completed the study. The flow chart of study participants is demonstrated in Fig. 1.
Baseline characteristics including sex, age, duration of diabetes, and medications used for controlling blood glucose, blood pressure, and lipid profile were not significantly different between the two groups (Table 2).
Moreover, anthropometric measures including height, weight, BMI, waist and hip circumferences, WHR, and physical activity (MET-min/week) were not significantly different between the two study groups at the baseline as well as the end of the trial (Table 3).
Dietary factors and nutrient intake, including total energy, dietary carbohydrate, cholesterol, vitamin C, alpha-carotene, and selenium (Se), total fat, MUFA, and PUFA intake, did not show significant differences within or between the groups during the study (Table 3). None of the baseline variables showed any significant difference between groups, asides from HGA1C (P = 0.015). Plasma NO showed no significant differences between and within the groups, although the BJ group’s mean differences increased much more noticeably after the 12 weeks compared with the control group (8.57 ± 23.93 in the BJ group vs. 2.42 ± 15.97 in the control group).
There was a significant reduction in plasma insulin and HOMA-β in the control group (P = 0.014, P = 0.038, respectively) by the end of the study (insulin and HOMA-β mean difference were − 3.93 in the control group vs. − 1.48 in the BJ group, and − 0.59 in the control group vs. 0.03 in the BJ group, respectively). However, there was no significant change in HOMA-IR in the same time frame. Even though SBP had shown a decrease in the BJ group after 12 weeks of intervention (129.47 ± 14.32 vs.125.94 ± 15.83, P = 0.116), it proved to be insignificant. However, DBP faced a significant decrease in the BJ group (74.73 ± 16.78 vs. 72.36 ± 16.09, P = 0.046). HDL showed a marginally significant reduction at the end of the intervention in the control group (70.81 ± 11.24 vs. 65.44 ± 6.46, P = 0.058).
Based on the ANCOVA model, none of the mentioned differences remained significant. FPG, HOMA-IR, TC, TG, and LDL did not show any significant differences during the whole study (Table 4).
Discussion
The present study is the first randomized, parallel-group, open-labeled clinical trial designed to investigate the beneficial properties of total nutrient and bioactive compounds of concentrated BJ on glycemic control, blood pressure, and lipid profile in individuals with T2D.
Our findings showed that consumption of BJ beetroot juice had beneficial effects on glycemic measures and DBP in patients with T2D by inhibiting a decrease in insulin secretion, HOMA-β, and improving endothelial function. Adjustment for confounding factors, including baseline measures, did not provide the same results. Plasma NO concentrations were elevated after concentrated BJ supplementation.
The range of nitrate and bioactive compounds in industrial beetroot juice has been reported differently across many studies. This includes Bahadoran’s study [17] which reported the amount of nitrate from 388 to 3968 mg/l and Wruss’s study [22] which indicated large variations of the nitrate levels ranging from 10 to 2400 mg/l. The purpose of this study was to investigate the effect of consumed bioactive compounds from a concentrated BJ with the least amount of industrial interference. The amount of nitrate in beet juice for daily consumption in this study was lower than in previous studies, including Gilchrist et al. [18], Kerley et al. [13], and Singh et al. [12]. The lower nitrate level of the juice in this study could be the probable factor behind the variations in statistics, reports, and results.
NO and other bioactive contents of beetroot juice regulate insulin and glucose homeostasis [4]. NO increases insulin secretion and sensitivity by enhancing pancreatic blood flow, beta cell viability, activation of guanylyl cyclase, and cGMP pathways [4, 7, 31]. It also increases the Glut4 gene expression/nitrosation and insulin-independent translocation of GLUT4 [7, 31]. NO inhibits the production of ROS in adipocytes and increases the dephosphorylation activity of PK1B. And then, through activating the PI3K/PKB signal cascade, enhances eNOS phosphorylation and NO production [7, 31], subsequently increases insulin-dependent translocation of GLUT4 [4, 7, 11, 31]. Bioactive compounds in the beetroot are responsible for AMPK activating as well as reduction of carbohydrate digestion and intestinal glucose absorption. They suppress postprandial glucose response and improve insulin sensitivity/secretion. GLUT4, along with AMPK, improves insulin sensitivity/resistance and glucose uptake [4].
In support of our findings, Gilchrist et al. reported that administration of 250 ml beetroot juice (containing 500 mg NO3) vs. NO3-depleted beetroot juice for 2 weeks did not improve insulin sensitivity in T2D patients, unlike an increase in plasma nitrite and nitrate concentration [18]. Bahadoran et al. also assessed the effect of daily supplementation of 5 g of beet juice powder containing about 250 mg of nitrate in people with T2DM for 24 weeks. This study also did not find any positive effects on metabolic variables compared to the placebo group [17]. This finding could be a result of decreased NO3 to NO2 bioconversion capacity in T2DM. Similarly, the study of Kapil et al. on hypertensive older adults and Fuchs et al. [32] on obese, insulin-resistant patients showed that daily consumption of 250 ml beetroot juice vs. NO3-depleted beetroot juice and water, respectively, resulted in no effect on fasting glucose, HbA1c, postprandial glucose, and insulin response [20]. Short time duration of the interventions or small sample sizes could affect the results. In contrast, an animal experiment conducted by Hong Jiang et al. [31] showed that administering 50 mg/l sodium nitrite for 4 weeks significantly improved fasting glucose and reduced insulin levels in db/db diabetic mice.
Hong Jiang et al. showed that NO stimulates GLUT4 translocation independent of insulin and improves insulin signaling through the restoration of NO-dependent nitrosation of GLUT4 signaling translocation [31]. Wootton-Beard et al. examined the postprandial glucose and insulin responses following supplementation with 225 ml beetroot juice (containing 990 mg NO3) compared with a macronutrient matched control beverage in a group of sixteen healthy adults. The results indicated a significantly lowered postprandial insulin response in 0–60 min and a significantly lowered glucose response in 0–30 min in the beetroot juice consuming group compared with the control. Any bioactive ingredients found in the beetroot juice may be responsible for the observed differences in the postprandial insulin responses [33]. The observed differences may be due to dissimilar examined populations, confounding factors, and differences in the designs of studies.
Bioactive contents of beetroot improve endothelial function and have blood-pressure-lowering effects. NO reduces vascular stiffness, ROS production, and oxidative stress. It also increases antioxidant and defensive enzyme activity [4]. NO also activates the SGC-cGMP pathway, directly decreases the angiotensin II type I receptor gene expression, improves the renal blood flow and vascular relaxation, and changes the Na/water retention [4]. Other bioactive ingredients suppress Cox-2 as pro-inflammatory prostaglandins, reduce the inflammatory markers including NF-κB, improve the endothelial function, and hence reduce the blood pressure [4]. According to the literature review, one study has assessed the effects of the dietary nitrate on BP in individuals with T2DM and reported no positive effect following beetroot juice supplementation [18]. These results are in agreement with our study. The peak of plasma nitrate concentration is expected to occur between 2 and 3 h after the consumption of beetroot juice. This may explain the lack of reduction in blood pressure (BP) after examination—approximately 16 h after the peak [18, 23].
Several studies have indicated no effects of beetroot juice on blood pressure in hypertensive, overweight, obese middle-aged, and older adult individuals [34,35,36,37]. The reported results may be due to a short-term dietary nitrate supplementation. The studies assessed the effects of daily beetroot juice supplementation on BP in nondiabetic subjects, including a study on hypertensive, untreated adults [14], and another study on healthy subjects [38], which reported an improvement in BP.
The noticeable increase in NO bioavailability could be the underlying mechanism in BP decreasing [4, 23], which could be disrupted by several complications in T2DM, including diminished vascular reactivity due to vascular aging, a subsequent decreased NO3 to NO2 bioconversion capacity, particularly impaired responsiveness to NO [4, 18, 23], high blood glucose and lipid levels, accumulation of advanced glycation end products, increased oxidative stress and NF-kB, as well as concomitant medications [18]. Besides, the techniques used to measure BP [18] and subjects’ ages [39] could explain the contrasts in the reported results. Animal model studies suggest that nitrate targets a novel pathway that enhances fat metabolism and energy utilization and eventually decreases plasma lipid levels [13]. In contrast to the current study, 8 weeks of nitrate intake (100 mg/L in drinking water) reduced LDL cholesterol in normal and diabetic rats compared to normal and diabetic rats without nitrate [15]. Furthermore, it has been reported that total and LDL cholesterol levels in patients suspected of coronary artery disease (CAD) are negatively correlated with plasma nitrate/nitrite levels[40]. In a pilot study on untreated hypertensive adults, daily consumption of 250 ml row beetroot juice (RBJ) compared with 250 g cooked beetroot significantly decreased non-HDL-C, LDL-C, and total TC [14]. It is suggested that the total antioxidant capacity (TAC) increase in RBJ-consuming subjects are responsible for the decline in lipid peroxidation [14, 41]. In a similar instance, a 2-week daily supplementation of controlled and uncontrolled hypertensive patients with 140 ml nitrate-rich beetroot juice (containing 800 mg NO3) led to increased NO bioavailability in both groups and reduced serum LDL-C only in uncontrolled hypertensive patients [13]. Our observation was consistent with any differences in plasma lipids, including TC, HDL, LDL, and TG. In agreement with our study, Velmurugan et al. reported that daily consumption of 250 ml beetroot juice (containing 370 mg NO3) vs. NO3-depleted beetroot juice does not affect oxidized-LDL level, as a marker of oxidative stress, in hypercholesterolemia patients [19]. It has been demonstrated that hypercholesterolemia reduces the availability of an NO substrate. It is noticeable that our research is the first human study that investigated concentrated BJ supplementary effect on plasma lipid (lipoprotein fractions) profile in T2DM patients thus, we cannot get insight into the underlying mechanism, and more human studies are needed. Considering Betalain’s effects on improving the lipid profile and its other biological effects, it could be a highly sensitive factor that should be considered in future studies and examinations [22].
Conclusions
The current study is a randomized, open-labeled trial, which has been designed for the first time to assess the effects of concentrated BJ on anthropometric measures, glycemic control, blood pressure, and lipid profile in T2D patients. This study encountered some drawbacks due to a lack of placebos for the control group. On the other hand, our study period was simultaneous with the COVID-19 pandemic. Due to a high drop-out rate observed during the study period, the sample size was decreased, which resulted in limitations with the number of patients. We could not achieve our IRCT calculated sample size; thus, the sample size was calculated with a smaller population. In conclusion, our findings suggest that daily consummation of 24 ml concentrated BJ does not affect FPG, insulin, HOMA-IR, HOMA-β, TC, LDL, HDL, TG, and BP. Hence, it is suggested that this study be repeated with larger sample size and different variations of beetroot juice containing various concentrations of bioactive compounds, mainly nitrate and betalains.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- WHR:
-
Waist to hip ratio
- MET:
-
Metabolic equivalent of tasks
- NO:
-
Nitric oxide
- FPG:
-
Fasting blood sugar
- HbA1c:
-
Hemoglobin A1c
- HOMA-IR:
-
Homeostasis model assessment for insulin resistance
- HOMA-β:
-
Homeostasis model assessment of β-cell function
- BP:
-
Blood pressure
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TC:
-
Total cholesterol
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoproteins
- TG:
-
Triglycerides
- Glut4:
-
Glucose transporter type 4
- ROS:
-
Reactive oxygen species
- PKB:
-
Protein-tyrosine phosphatase 1B
- Akt:
-
Protein kinase B (PKB), also known as Akt
- PI3K:
-
Phosphoinositide 3-kinases
- eNOS:
-
Endothelial NO synthase
- SGC:
-
Soluble guanylate cyclase
- cGMP:
-
Cyclic guanosine monophosphate
- COVID-19:
-
Coronavirus disease 2019
References
WHO O (2016) World health statistics monitoring health for the SDGs sustainable development goals Geneva. World Health Organization 2016:2016
Hodaei H et al (2019) The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: a randomized, double-blind clinical trial. Diabetol Metab Syndr 11(1):1–8
Katz DL (2014) Diet and diabetes: lines and dots. J Nutr 144(4):567S-570S
Mirmiran P et al (2020) Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr Metab 17(1):1–15
Chhikara N et al (2019) Bioactive compounds of beetroot and utilization in food processing industry: a critical review. Food Chem 272:192–200
Baião DD, da Silva DV et al (2017) Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. Food Additives 6(6)
Bahadoran Z et al (2015) Beneficial effects of inorganic nitrate/nitrite in type 2 diabetes and its complications. Nutr Metab 12(1):1–9
Hadipour E et al (2020) Biological effects of red beetroot and betalains: a review. Phytother Res 34(8):1847–1867
Clifford T et al (2015) The potential benefits of red beetroot supplementation in health and disease. Nutrients 7 (4):2801–2822. (PubMed Abstract| Publisher Full Text| Free Full Text)
Bahadoran Z et al (2017) Vitamin C intake modify the impact of dietary nitrite on the incidence of type 2 diabetes: a 6-year follow-up in Tehran lipid and glucose study. Nitric Oxide 62:24–31
Schiffer TA et al (2020) Modulation of mitochondria and NADPH oxidase function by the nitrate-nitrite-NO pathway in metabolic disease with focus on type 2 diabetes. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Dis 165811
Singh A et al (2015) Beetroot juice supplementation increases high density lipoprotein-cholesterol and reduces oxidative stress in physically active individuals. J Pharm Nut Sci 5(3):179–185
Kerley CP, Dolan E, Cormican L (2017) Nitrate-rich beetroot juice selectively lowers ambulatory pressures and LDL cholesterol in uncontrolled but not controlled hypertension: a pilot study. Irish J Med Sci 186(4):895–902
Asgary S et al (2016) Improvement of hypertension, endothelial function and systemic inflammation following short-term supplementation with red beet (Beta vulgaris L.) juice: a randomized crossover pilot study. J human hypertension 30(10):627–632
Khalifi S et al (2015) Dietary nitrate improves glucose tolerance and lipid profile in an animal model of hyperglycemia. Nitric Oxide 44:24–30
Ohtake K et al (2017) Dietary nitrite reverses features of postmenopausal metabolic syndrome induced by high-fat diet and ovariectomy in mice. American Journal of Physiology-Endocrinology and Metabolism 312(4):E300–E308
Bahadoran Z et al (2021) Effect of inorganic nitrate on metabolic parameters in patients with type 2 diabetes: a 24-week randomized double-blind placebo-controlled clinical trial. Nitric Oxide 107:58–65
Gilchrist M et al (2013) Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radical Biol Med 60:89–97
Velmurugan S et al (2016) Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr 103(1):25–38
Kapil V et al (2015) Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65(2):320–327
Altman DG et al (2001) The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 134(8):663–694
Wruss J et al (2015) Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J Food Compos Anal 42:46–55
Jajja A et al (2014) Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr Res 34(10):868–875
Bahadoran Z et al (2019) Estimation and validation of dietary nitrate and nitrite intake in Iranian population. Iran J Public Health 48(1):162
Behrouz V et al (2020) The effect of crocin supplementation on glycemic control, insulin resistance and active AMPK levels in patients with type 2 diabetes: a pilot study. Diabetol Metab Syndr 12(1):1–9
Roshan H et al (2018) Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: a randomised clinical trial. Br J Nutr 119(3):250–258
Ha CH et al (2015) Effects of combined exercise on HOMA-IR, HOMA β-cell and atherogenic index in Korean obese female. Sport Sciences for Health 11(1):49–55
Olumese FE, Oboh HA (2016) Antioxidant and antioxidant capacity of raw and processed Nigerian beetroot (Beta vulgaris). Nigerian Journal of Basic and Applied Sciences 24(1):35–40
Ravichandran K et al (2012) The effect of different processing methods on phenolic acid content and antioxidant activity of red beet. Food Res Int 48(1):16–20
Organization INS (2015) Vegetables and vegetable products -determination of nitrate and/or nitrite content part7-continuous flow method for the determination of nitrate content after cadmium reduction. 1st Edition
Jiang H et al (2014) Dietary nitrite improves insulin signaling through GLUT4 translocation. Free Radical Biol Med 67:51–57
Fuchs D et al (2016) Impact of flavonoid-rich black tea and beetroot juice on postprandial peripheral vascular resistance and glucose homeostasis in obese, insulin-resistant men: a randomized controlled trial. Nutr Metab 13(1):1–10
Wootton-Beard PC et al (2014) Effects of a beetroot juice with high neobetanin content on the early-phase insulin response in healthy volunteers. J nutritional sci 3
Bondonno CP et al (2015) Absence of an effect of high nitrate intake from beetroot juice on blood pressure in treated hypertensive individuals: a randomized controlled trial. Am J Clin Nutr 102(2):368–375
Lara J et al (2015) Effects of handgrip exercise or inorganic nitrate supplementation on 24-h ambulatory blood pressure and peripheral arterial function in overweight and obese middle age and older adults: a pilot RCT. Maturitas 82(2):228–235
Shepherd AI et al (2015) The effect of dietary nitrate supplementation on the oxygen cost of cycling, walking performance and resting blood pressure in individuals with chronic obstructive pulmonary disease: a double blind placebo controlled, randomised control trial. Nitric Oxide 48:31–37
Cermak NM et al (2012) No improvement in endurance performance after a single dose of beetroot juice. Int J Sport Nutr Exerc Metab 22(6):470–478
Vanhatalo A et al (2010) Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 299(4):R1121–R1131
Siervo M et al (2013) Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J Nutr 143(6):818–826
Tanaka S et al (1997) Plasma nitrite/nitrate level is inversely correlated with plasma low-density lipoprotein cholesterol level. Clin Cardiol 20(4):361–365
Sener G et al (2002) Effects of chard (Beta vulgaris L. var. cicla) extract on oxidative injury in the aorta and heart of streptozotocin-diabetic rats. J medicinal food 5(1):37–42
Acknowledgements
This study was supported by the National Nutrition and Food Technology Research Institute of Iran. The authors would like to express their gratitude to the subjects for their participation and cooperation in this research; Jahan Medical Lab, Tehran, Iran for professional support and Takdaneh, Inc., Marand, Iran for preparing the beetroot juices. The authors wish to acknowledge Mr. Barbod Safari for his critical editing of the English grammar and syntax of the manuscript.
Funding
Shahid Beheshti University of Medical Sciences supported this research. The funder has no role in study design, data collection, and analysis as well as manuscript publication.
Author information
Authors and Affiliations
Contributions
LK and GS conceptualized and designed the study and wrote the manuscript; VB and GE collected data; LK, GE, and TR provided the study administration works. LK, GS, SE, and MH interpreted the data, provided professional comments, and critically revised the manuscript for intellectual content and data accuracy. All authors had access to the study data and reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Shahid Beheshti University of Medical Sciences has approved the study protocol (IR.SBMU.nnftri.Rec.1399.035). A written informed consent form was signed and dated by subjects and investigators at the beginning of the study (in Persian). Participation was free, and a patient could withdraw, anytime they feel unable to continue. The personal information of participants was kept secret before, during, and after the trial.
Consent for publication
A written consent to publish the information and data of the participants was obtained.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
karimzadeh, L., Sohrab, G., Hedayati, M. et al. Effects of concentrated beetroot juice consumption on glycemic control, blood pressure, and lipid profile in type 2 diabetes patients: randomized clinical trial study. Ir J Med Sci 192, 1143–1153 (2023). https://doi.org/10.1007/s11845-022-03090-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-03090-y