Abstract
Introduction
Butyrylcholinesterase (BChE), an important biomarker of exposure to anticholinesterases, varies its activity according to the intensity and duration of exposure to these agents. Their normal values may vary in different populations. It is important to determine the reference values for the local population, mostly black/brown.
Objective
The objective was to investigate the baseline values of BChE activity in a sample of the Salvador city population (Bahia, Brazil), evaluating the sociodemographic characteristics.
Method
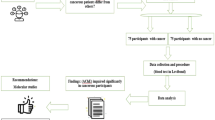
A descriptive, quantitative study with a cross-sectional approach was carried out in 304 voluntary and healthy blood donors. BChE activity was determined using the integrated chemical system Dimension RxLMax and analyses of sociodemographic characteristics were performed.
Results
For the 304 participants (18 to 67 years old), BChE activity values range were 7.4 to 19.8 U/mL (male) and 6.0 to 19.6 U/mL (female), without significant inter-racial differences (p = 0.986; Mann–Whitney). The participates were predominantly black (44.7%) and brown (40.5%), with higher levels of BchE activity in males (64.8%) (p-value = 0.01) than females (35.2%). There was no relationship between alcohol use and lower BChE activity (p = 0.725, Mann–Whitney). Women using hormonal contraceptives had a median activity 9.2% lower than the non-users.
Conclusion
Despite the high miscegenation and predominance of the black race in Salvador, contrary to what was expected, the sample did not show statistically significant intra-racial differences in BChE activity, being able to use the same reference values currently used, observing factors such as sex, use of contraceptives, and drinking alcohol.
Similar content being viewed by others
References
Makhaeva GF, Rudakova EV, Richardson RJ (2018) Investigation of the esterase status as a complex biomarker of exposure to organophosphorus compounds. biomedical chemistry: research and methods. Institute of Biochemistry 1(3):e00028. https://doi.org/10.18097/bmcrm00028
Alonzo HGA, Corrêa CL (2003) Praguicidas. In: OGA, Seizi. Fundamentos de toxicologia. São Paulo: Atheneu (2):437–458
Goodal R (2004) Cholinesterase: phenotyping and genotyping. Ann Clin Biochem 41(Pt 2):98–110. https://doi.org/10.1258/000456304322879971 (PMID: 15025799)
Johnson G, Moore SW (2012) Why has butyrylcholinesterase been retained? Structural and functional diversification in a duplicated gene. Neurochem Int 61(5):783–797. https://doi.org/10.1016/j.neuint.2012.06.016 (Epub 2012 Jun 28 PMID: 22750491)
Lockridge O (2015) Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther 148:34–46. https://doi.org/10.1016/j.pharmthera.2014.11.011 (Epub 2014 Nov 20 PMID: 25448037)
Delacour H, Dedome E, Courcelle S et al(2016) Butyrylcholinesterase deficiency. Ann Biol Clin (Paris) 74(3):279–85. English. https://doi.org/10.1684/abc.2016.1141 (PMID: 27237801)
Lockridge O (1990) Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. Pharmacol Ther 47(1):35–60. https://doi.org/10.1016/0163-7258(90)90044-3 (PMID: 2195556)
Chen VP, Gao Y, Geng L, Brimijoin S (2017) Butyrylcholinesterase regulates central ghrelin signaling and has an impact on food intake and glucose homeostasis. Int J Obes (Lond) 41(9):1413–1419. https://doi.org/10.1038/ijo.2017.123 (Epub 2017 May 22. PMID: 28529331; PMCID: PMC5585042)
Pope CN, Brimijoin S (2018) Cholinesterases and the fine line between poison and remedy. Biochem Pharmacol 153:205–216. https://doi.org/10.1016/j.bcp.2018.01.044 (Epub 2018 Jan 31. PMID: 29409903; PMCID: PMC5959757)
Carmona-Fonseca J (2006) Relación entre los niveles de colinesterasa y los grupos sanguíneos ABO y Rh. Acta Med Colomb 31(3):104–112
Caro-Gamboa LJ, Forero-Castro M, Dallos-Báez AE (2020) Cholinesterase inhibition as a biomarker for the surveillance of the occupational population exposed to organophosphorus pesticides. Ciencia y Tecnología Agropecuaria 21(3). https://doi.org/10.21930/rcta.vol21_num3_art:1562
Wood AJ (2001) Racial differences in the response to drugs–pointers to genetic differences. N Engl J Med 344(18):1394–1396. https://doi.org/10.1056/NEJM200105033441811 (PMID: 11336055)
Lockridge O, Norgren RB Jr, Johnson RC, Blake TA (2016) Naturally occurring genetic variants of human acetylcholinesterase and butyrylcholinesterase and their potential impact on the risk of toxicity from cholinesterase inhibitors. Chem Res Toxicol 29(9):1381–92. https://doi.org/10.1021/acs.chemrestox.6b00228 (Epub 2016 Aug 31. PMID: 27551784; PMCID: PMC5030680)
Vale NB, Delfino J (2003) Anestesia na população negra [Anesthesia in the afro-american population]. Rev Bras Anestesiol 53(3):401–18. Portuguese. https://doi.org/10.1590/s0034-70942003000300013 (PMID: 19475293)
Vale NB, Delfino J, Vale LFB (2003) O Conhecimento de Diferenças Raciais pode Evitar Reações Idiossincrásicas na Anestesia? Rev Bras Anestesiol 53(2):258–277
Jones DS (2013) How personalized medicine became genetic, and racial: Werner Kalow and the formations of pharmacogenetics. J Hist Med Allied Sci 68(1):1–48. https://doi.org/10.1093/jhmas/jrr046 (Epub 2011 Sep 10 PMID: 21908852)
Chautard-Freire-Maia EA, Carvalho RD, da Silva MC et al (1984) Frequencies of atypical serum cholinesterase in a mixed population of northeastern Brazil. Hum Hered 34(6):364–370. https://doi.org/10.1159/000153497 (PMID: 6510933)
Ceppa F, Gidenne S, Benois A et al (2002) Rapid identification of atypical variant of plasma butyrylcholinesterase by PCR. Clin Chem Lab Med 40(8):799–801. https://doi.org/10.1515/CCLM.2002.138 (PMID: 12392308)
Delacour H, Lushchekina S, Mabboux I et al (2014) Characterization of a novel BCHE “silent” allele: point mutation (p.Val204Asp) causes loss of activity and prolonged apnea with suxamethonium. PLoS One 9(7):e101552. https://doi.org/10.1371/journal.pone.0101552 (PMID: 25054547; PMCID: PMC4108472)
Goodall R, Association of Clinical Biochemists Analytical Investigations Standing Committee (2004) Cholinesterase: phenotyping and genotyping. Ann Clin Biochem 41(Pt 2):98–110. https://doi.org/10.1258/000456304322879971 (PMID: 15025799)
Howard TD, Hsu FC, Grzywacz JG et al (2010) Evaluation of candidate genes for cholinesterase activity in farmworkers exposed to organophosphorus pesticides: association of single nucleotide polymorphisms in BCHE. Environ Health Perspect 118(10):1395–9. https://doi.org/10.1289/ehp.0901764 (Epub 2010 Jun 8. PMID: 20529763; PMCID: PMC2957918)
McQueen MJ (1995) Clinical and analytical considerations in the utilization of cholinesterase measurements. Clin Chim Acta 237(1–2):91–105. https://doi.org/10.1016/0009-8981(95)06067-n (PMID: 7664482)
Lockridge O, Bartels CF, Vaughan TA et al (1987) Complete amino acid sequence of human serum cholinesterase. J Biol Chem 262(2):549–557 (PMID: 3542989)
Borges-Osório MR, Robinson WM (2001) Genética humana. Porto Alegre: Artmed (3):243–244
Ramaiah M, Prudhivi RK (2020) Pseudocholinesterase deficiency in an Indian community. Journal of Pharmacy Practice and Community Medicine 3(1):27–30. https://doi.org/10.5530/jppcm.2017.1.6
Macqueen J, Plaut D (1973) A review of clinical applications and methods for cholinesterase. Am J Med Technol 39(7):279–287 (PMID: 4354600)
Henry JB (1989) Diagnósticos clínicos e conduta terapêutica por exames laboratoriais. Ed Manole 1(16):414–416
Janeway CA (1980) Review: biological function of cholinesterase. Clin Biochem 13(6):239–243
Negrão AB, Pereira AC, Guindalini C et al (2013) Butyrylcholinesterase genetic variants: association with cocaine dependence and related phenotypes. PLoS ONE 8(11):e80505. https://doi.org/10.1371/journal.pone.0080505.PMID:24312228;PMCID:PMC3842332
Câmara SA, Silva I, Pontes ER, Barbosa AM (2012) Exposição a agrotóxicos: determinação dos valores de referência para colinesterase plasmática e eritrocitária. Brasília Med 49(3):163–169
Siqueira MEPB, Fernícola NAGG, Borges EL (1978) Determinação de níveis normais de colinesterase plasmática e eritrocitária. Rev Saúde Pública São Paulo 12(3). https://doi.org/10.1590/S0034-89101978000300008.
Salzano FM, Sans M (2014) Interethnic admixture and the evolution of Latin American populations. Genet Mol Biol 37(1 Suppl):151–170. https://doi.org/10.1590/s1415-47572014000200003.PMID:24764751;PMCID:PMC3983580
Petrucelli JL, Saboia AL (2013) Características Étnico-raciais da População: classificações e identidades. IBGE Rio de Janeiro 2:208
Brasilde Geografia e Estatística - IBGE. Censo Demográfico, IB (2010) Características gerais da população, religião e pessoas com deficiência. Rio de Janeiro 2010:66
Gal EM, Roth E (1957) Spectrophotometric methods for determination of cholinesterase activity. Clin Chim Acta 2(4):316–326. https://doi.org/10.1016/0009-8981(57)90009-8 (PMID: 13473097)
Siemens Healthcare Diagnostics Ltd (2018) Dimension clinical chemistry system. Flex reagent cartridge. Instruction manual. Camberley
US Department of Health and Human Services Food and Drug Administration (2019) Draft guidance document: M10 bioanalytical method validation. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/m10-bioanalytical-method-validation. Accessed 9 Jul 2021
Sidell FR, Kaminskis A (1975) Influence of age, sex, and oral contraceptives on human blood cholinesterase activity. Clin Chem 21(10):1393–1395 (PMID: 1157304)
Vásquez L, Osorio J (2000) Variación de la Actividad de la Enzima Butirilcolinesterasa en Usuarias de Anticonceptivos Hormonales. An Fac Med (Perú) 61(4):271–277
Whittaker M, Charlier AR, Ramaswamy S (1971) Changes in plasma cholinesterase isoenzymes due to oral contraceptives. J Reprod Fertil 26(3):373–375. https://doi.org/10.1530/jrf.0.0260373 (PMID: 5569652)
Huang A, Chang B, Sun Y et al (2017) Disease spectrum of alcoholic liver disease in Beijing 302 hospital from 2002 to 2013: a large tertiary referral hospital experience from 7422 patients. Medicine (Baltimore) 96(7) (PMID: 28207552; PMCID: PMC5319541)
Hemantha Kumara DS, Muralidhara Krishna CS, Vishwanath H (2018) A comparative study in assessing the usefulness of serum cholinesterase, high sensitivity C-reactive protein with Liver Function Tests in Alcoholic Liver disease. Indian J Med Biochem 22(2):147–153. https://doi.org/10.5005/jp-journals-10054-0073
Giolo SR, Soler JM, Greenway SC et al (2012) Brazilian urban population genetic structure reveals a high degree of admixture. Eur J Hum Genet 20(1):111–6. https://doi.org/10.1038/ejhg.2011.144 (Epub 2011 Aug 24. PMID: 21863058; PMCID: PMC3234512)
Queiroz EM, Santos AM, Castro IM et al (2013) Genetic composition of a Brazilian population: the footprint of the Gold Cycle. Genet Mol Res 12(4):5124–5133. https://doi.org/10.4238/2013.October.29.6 (PMID: 24301772)
Sánchez LH, Medina OM, Gómez G et al (2015) Laboratory genetic-based reference values for cholinesterase activity in a Colombian population: a step forward in personalized diagnostics. Biomedica 35 Spec:20–9. https://doi.org/10.1590/S0120-41572015000500003 (PMID: 26535739)
Simpson NE (1996) Factors influencing cholinesterase activity in a Brazilian population. Am J Hum Genet 18(3):243–52 (PMID: 5944418; PMCID: PMC1706078)
Jiménez-Díaz M, Schosinsky-Nevermann K (2000) Valores de referencia de colinesterasa plasmática y eritrocítica en población costarricense: comparación del desempeño clínico de ambas enzimas. Rev costarric cienc méd 21(3–4):117–126
Carmona-Fonseca J (2006) Correlación y conversión entre valores de colinesterasa eritrocitaria medida con las técnicas de Michel y EQM. Biomedica 26(4):546–555. https://doi.org/10.7705/biomedica.v26i4.324
Zlatković M, Krstić N, Subota V et al (2017) Determination of reference values of acetyl and butyryl cholinesterase activities in Serbian healthy population. Vojnosanit Pregl 74(8):736–741. https://doi.org/10.2298/VSP160303101Z
Karasova JZ, Maderycova Z, Tumova M et al (2017) Activity of cholinesterases in a young and healthy middle-European population: relevance for toxicology, pharmacology and clinical praxis. Toxicol Lett 5(277):24–31. https://doi.org/10.1016/j.toxlet.2017.04.017 (Epub 2017 Apr 30 PMID: 28465191)
Worek F, Schilha M, Neumaier K et al (2016) On-site analysis of acetylcholinesterase and butyrylcholinesterase activity with the ChE check mobile test kit-determination of reference values and their relevance for diagnosis of exposure to organophosphorus compounds. Toxicol Lett 13(249):22–28. https://doi.org/10.1016/j.toxlet.2016.03.007 (Epub 2016 Mar 29 PMID: 27033775)
Carmona-Fonseca J, Henao S, Garcés R (2000) Valores de referencia de actividad colinesterásica sanguínea en población laboral activa no expuesta a plaguicidas inhibidores de colinesterasa. Rev Fac Nal Sal Públ (Medellín) 18:55–72
Jensen FS, Skovgaard LT, Viby-Mogensen J (1995) Identification of human plasma cholinesterase variants in 6,688 individuals using biochemical analysis. Acta Anaesthesiol Scand 39(2):157–162. https://doi.org/10.1111/j.1399-6576.1995.tb04035.x
Lepage L, Schiele F, Gueguen R, Siest G (1985) Total cholinesterase in plasma: biological variations and reference limits. Clin Chem 31(4):546–550 (PMID: 3978785)
Lockridge O, Masson P (2000) Pesticides and susceptible populations: people with butyrylcholinesterase genetic variants may be at risk. Neurotoxicology 21(1–2):113–26 (PMID: 10794391)
Ichihara K, Ozarda Y, Barth JH et al (2017) A global multicenter study on reference values: 1. Assessment of methods for derivation and comparison of reference intervals. Clin Chim Acta 467:70–82. https://doi.org/10.1016/j.cca.2016.09.016 (Epub 2016 Sep 22. PMID: 27666761)
Küçükosman G, Pişkin Ö, Hancı V et al (2018) Pseudocholinesterase levels in patients under electroconvulsive therapy. Saudi Med J 39(1):103–106. https://doi.org/10.15537/smj.2018.1.21307 (PMID: 29332117; PMCID: PMC5885109)
Abou-Hatab K, O’Mahony MS, Patel S, Woodhouse K (2001) Relationship between age and plasma esterases. Age Ageing 30(1):41–45. https://doi.org/10.1093/ageing/30.1.41 (PMID: 11322671)
Hosseini J, Firuzian F, Feely J (1997) Ethnic differences in the frequency distribution of serum cholinesterase activity. Ir J Med Sci 166(1):10–2. https://doi.org/10.1007/BF02939767 (PMID: 9057423)
Acknowledgements
The authors thank the institutions Toxicological Information and Assistance Center of Bahia (CIATox-BA), the Secretariat of Health of the State of Bahia (SESAB), the Federal University of Bahia (UFBA), and the Hematology and Hemotherapy Foundation of Bahia (HEMOBA) who kindly authorized the access and use of their structures, necessary to carry out this work. This research had the support of Anicele de Jesus and Leonardo Buffone in collecting material, Agnaldo de Souza Orrico in the statistical analysis, and Siemens Helthcare in providing kits for determination of dosages of BChE activity. The authors would also like to thank each of the collaborators and donors participating in the research, without whom it would not materialize.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Conceição Filho, J.N., dos Santos, I.C., Gonçalves, D.P.d. et al. Black and non-black population: investigation of the difference in butyrylcholinesterase activity in a healthy population in Salvador, Bahia. Ir J Med Sci 192, 1311–1319 (2023). https://doi.org/10.1007/s11845-022-03087-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-03087-7