Abstract
Objective
Audit is a recognised tool for evaluating the performance and improving the quality of health services. In Ireland and the UK, clear resources are available outlining audit elements. This study was undertaken to evaluate paediatric audits published from 2007 to 2020 to determine the adherence level to the definition of audit and to assess the quality of audit standards.
Design
PUBMED, MEDLINE and CINAHL databases were searched to identify relevant articles published in the English language. Each was reviewed to assess whether the following criteria were met: (1) a paediatric healthcare topic was described, (2) practice was reviewed, (3) the standard was specified, (4) an intervention was made and data collection was repeated to assess improvement. The quality of the standard for each true audit was graded utilising the Oxford Centre for Evidence-Based Medicine Levels of Evidence.
Results
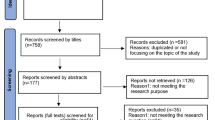
Of 1230 published paediatric healthcare articles reviewed, 144 (11.4%) fulfilled the full criteria of an audit. Sixty-three (43.8%) true audits used the highest quality of evidence (level 1a and 1b), predominantly international or national guidelines. Fifty-six (38.9%) audits used the lowest quality of evidence (level 5), predominantly expert opinion.
Conclusions
There is a mismatch between the common usage of the term audit, and the definition, despite its incorporation into training curricula and institutional support. Many articles published as audits do not adhere to the definition of audit. There are variable levels of evidence supporting the standards utilised in published true audits.

Similar content being viewed by others
Change history
23 July 2022
A spelling error in author name Michael B. O’Neill has been updated.
References
Potter J, Fuller C, Ferris M (2010) Local clinical audit: handbook for physicians. Royal College of Physicians
Standing committee on Postgraduate Medical Education (1989) Medical audit-the educational implications. SCOMPE, London
Johnston G, Crombie IK, Davies HT et al (2000) Reviewing audit: barriers and facilitating factors for effective clinical audit. Qual Health Care 9(1):23–36. https://doi.org/10.1136/qhc.9.1.23.PMID:10848367;PMCID:PMC1743496
Faculty of Paediatrics (2021) Basic speciality training in paediatrics curriculum, 2nd edn. Ireland: RCPI, pp 14:19
Rcpi.ie (2021) CPD explained. [online] Available at https://www.rcpi.ie/professional-competence/information-for-enrolled-doctors/cpd-explained/. Accessed 18 July 2021
Partnership HQI (2016) Guide to involving junior doctors in clinical audit and quality improvement. HQIP, London
O'Gorman CS, Ziedan Y, O’Neill MB (2007) An evaluation of Medline published paediatric audits from 1966 to 1999. Arch Dis Child 92(4):309–311
Cebm.ox.ac.uk (2021) Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009) — Centre for Evidence-Based Medicine (CEBM), University of Oxford. [online] Available at https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009. Accessed 28 July 2021
National Review of Clinical Audit Working Group (2019) National Review of Clinical Audit Report 2019, 1st edn. HSE, Ireland
National Centre for Clinical Excellence (2002) Principle for Best Practice in Clinical Audit. Radcliffe Medical Press, United Kingdom
Kumar P, Hashmi Y, Morad R et al (2020) Clinical Audit Platform for Students (CAPS): a pilot study. Postgraduate Medical Journal. pp postgradmedj-2020–138426
Acknowledgements
We thank Ms. Julia Reynolds, the Medical Information Specialist, Mayo University Hospital, Ireland, for her invaluable help with searching and retrieving the articles.
Author information
Authors and Affiliations
Contributions
All six authors made contributions to the design of the study and were involved in the acquisition of data, drafting of the manuscript and final approval of the version to be published. All named authors have no conflict of interest, financial or otherwise.
Corresponding author
Ethics declarations
Ethical statement
This study evaluated published paediatric articles and no individual patient data was assessed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
What is already known on this topic?
• Audit is viewed as an essential part of practicing medicine.
What does this study add?
• There is a mismatch between the common usage of the term audit, and the definition, despite its incorporation into training curricula and institutional support.
• Evidence supporting standards used in published paediatric audits comes from varied sources—from high-quality international guidelines to low-quality local policies.
• Audit impact could be enhanced if the quality of the utilised standard was described by the authors.
Appendices
Appendix 1. Oxford centre for evidence-based medicine: Levels of evidence (March 2009)
Level
-
1a Systematic review (with homogeneity*) of RCTs, inception cohort studies, level 1 diagnostic studies, prospective cohort studies or economic studies.
-
1b Individual RCT (with narrow confidence interval), individual inception or prospective cohort study with > 80% follow-up, CDR validated in a single population.
-
1c All or none, all or none case series.
-
2a Systematic review (with homogeneity) of cohort studies or level 2 diagnostic studies.
-
2b Individual cohort study including low-quality RCT, retrospective cohort study.
-
2c “Outcomes” research, ecological studies.
-
3a Systematic review (with homogeneity) of case–control studies.
-
3b Individual case–control study, non-consecutive study.
-
4 Case series, poor quality cohort and case–control studies.
-
5 Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles”.
Appendix 2. Top ten highest impact paediatric journals as of July 2021, compiled using the Journal Citation Reports database
Journal (descending impact) | Audit criteria mentioned in submission guidelines | Audit mentioned in submission guidelines |
|---|---|---|
JAMA Pediatrics | No | No |
Lancet Child & Adolescent Health | No | No |
Journal Of The American Academy Of Child And Adolescent Psychiatry | No | No |
Pediatrics | No | No |
Pediatric Allergy And Immunology | No | No |
Archives Of Disease In Childhood-Fetal And Neonatal Edition | No | Yes |
Developmental Medicine And Child Neurology | No | No |
Journal Of Adolescent Health | No | No |
Pediatric Diabetes | No | No |
European Child & Adolescent Psychiatry | No | No |
Rights and permissions
About this article
Cite this article
Larkin, G.C., Mulroy, S.A., Nabialek, T. et al. The quality of published paediatric audits and their standards. Ir J Med Sci 192, 1277–1280 (2023). https://doi.org/10.1007/s11845-022-03082-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-03082-y