Abstract
Background
Characterizing the post-COVID health conditions is helpful to direct patients to appropriate healthcare.
Aims
To describe the presence of symptoms in COVID-19 patients within 6 months after diagnosis and to investigate the associated factors in terms of reporting symptoms.
Methods
Data of DEU-COVIMER (a telephone interview-based COVID-19 follow-up center established in a tertiary care hospital) was analyzed for SARS-CoV-2 RNA positive participants aged ≥ 18 years from November 1st, 2020, to May 31st, 2021. Symptom frequencies were stratified by demographic and clinical characteristics at one, three, and 6 months after diagnosis. With the patients who had symptoms at baseline, generalized estimating equations were applied to identify the factors associated with reporting of symptoms.
Results
A total of 5610 patients agreed to participate in the study. Symptom frequency was 37.2%, 21.8%, and 18.2% for the first, third, and sixth months. Tiredness/fatigue, muscle or body aches, and dyspnea/difficulty breathing were the most common symptoms in all time frames. In multivariate analysis, older age, female gender (odds ratio OR 1.74, 95% confidence interval 1.57–1.93), bad economic status (OR 1.37, 1.14–1.65), current smoking (OR 1.15, 1.02–1.29), being fully vaccinated before COVID-19 (OR 0.53, 0.40–0.72), having more health conditions (≥ 3 conditions, OR 1.78, 1.33–2.37), having more symptoms (> 5 symptoms, OR 2.47, 2.19–2.78), and hospitalization (intensive care unit, OR 2.18, 1.51–3.14) were associated with reporting of symptoms.
Conclusions
This study identifies risk factors for patients who experience post-COVID-19 symptoms. Healthcare providers should appropriately allocate resources prioritizing the patients who would benefit from post-COVID rehabilitation.
Similar content being viewed by others
Introduction
As of date, the coronavirus disease 2019 (COVID-19) pandemic has been going on for more than 2 years. Although the community mitigation strategies have been the main subject from the very beginning, new variants continue to change approaches to pandemic control. The omicron variant of severe acute respiratory virus 2 (SARS-CoV-2) spread faster than any previous variants, and as of February 2022, the world faces the highest daily number of new cases. Since the high number of people continues to be infected, efforts on measuring the long-term clinical impacts of the disease also will continue.
The long-term clinical effects of COVID-19 are referred to a general term conceptualized as post-COVID conditions. The post-COVID conditions first arose from patient-led notifications in March 2020. Patients who had overwhelmed symptoms began to share their experiences on social media which attracted the attention of newspapers and researchers [1]. Thus, discussions around post-COVID experiences sparkled new terminologies such as “long-haulers” and “long-COVID” [1, 2]. Early studies limited to post-hospitalized settings reported that 87% of the patients had at least one symptom in a mean of 60 days of monitoring [3], and at the sixth month, 76% of the patients were still symptomatic [4]. In the outpatient setting, studies revealed a range of 32 to 53% symptom frequency for different lengths of follow-ups [5, 6]. Fatigue, dyspnea, body aches, and loss of taste or smell were the frequently reported symptoms [3,4,5,6,7].
In parallel with rapidly growing literature on the topic, post-COVID conditions were common [8, 9], but there was a wide range of definitions including ongoing symptoms, relapsing symptoms, new clinical situations, new onset of a disease, or delayed return to usual health [2, 8,9,10,11]. In response to heterogeneity in the studies and the absence of a single-case definition, the World Health Organization held a series of meetings to facilitate global discussion on the topic. Currently, the post-COVID condition was defined as persistence of symptoms or new onset of symptoms after recovery without another explanation for at least 2 months. Symptoms may have fluctuating or relapsing nature which affects general health and quality of life every day [12].
Data obtained by the continuous monitoring of COVD-19 patients can help to develop clinical management strategies and direct the patients who experience post-COVID conditions to appropriate country-specific healthcare. Therefore, we aimed to characterize the presence of symptoms that may be associated with COVID-19 within the 6 months of follow-up after diagnosis and to investigate the associated baseline factors contributing to the reporting of symptoms.
Methods
Study design and DEU-COVIMER protocol
This prospective cohort study was conducted by Dokuz Eylul University Hospital COVID-19 follow-up center (DEU-COVIMER). Dokuz Eylul University Hospital is in the southwest region of Izmir, the third-largest city in Turkey with approximately 4.5 million population. The hospital has been a designated pandemic public hospital and people could visit the outpatient COVID-19 policlinic or emergency care unit with or without a referral. DEU-COVIMER was established in January 2021 to gain knowledge about the long-term health outcomes of COVID-19 patients by monitoring the patients with a multidisciplinary approach.
Prior to initiating data collection, we reviewed the available literature on possible long-term effects of COVID-19 and data collection methods developed by international COVID-19 working groups such as Respiratory and Emerging Infection Consortium (ISARIC) and the post-hospitalization COVID-19 study (PHOSP-COVID) [13, 14]. The questionnaires were designed by public health and epidemiology experts in the institution.
At the 1st, 3rd, and 6th months after the first positive test date, pre-trained DEU-COVIMER staff interviewed patients by telephone. The staff made at least five attempts until the end of the working hour to contact all the patients. The measurements at the 1st, 3rd, and 6th month included general health information (mortality, hospital admission), COVID-19 like-symptom check (fatigue, body aches, dyspnea, loss or change of smell and taste, etc.), health-related quality of life-EuroQol five-dimension three-level (EQ-5D-3L; mobility, self-care, usual activities, pain and discomfort, anxiety, and depression), and vaccination history. Additionally, the presence of healthcare utilization or newly diagnosed diseases were interviewed at the 3rd and 6th months. All participants provided oral informed consent before starting the telephone interview regarding the collection of data.
Study participants and variables
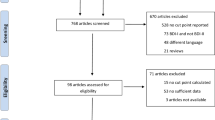
We invited patients aged ≥ 18 years to participate in the study who had a first positive reverse transcriptase-polymerase chain reaction (RT-PCR) test for SARS-CoV-2 from November 1st, 2020, to May 31st, 2021. DEU-COVIMER became fully operational on January 11, 2021, so we established two cohorts. To catch the subsequent follow-up points in the 1st, 3rd, and 6th months, we recruited the patients who tested positive for SARS-CoV-2 after November 30, 2020, as the main cohort in the study (December 2020–May 2021 cohort). Because the first-month monitoring was already missed for the people diagnosed before December 1st, 2020, we only interviewed them in the 3rd and 6th months (November 2020 cohort). In total, 6701 people tested positive for SARS-CoV-2 RNA between November 1st, 2020, and May 31st, 2021. We aimed to reach all patients; therefore, sample size was not calculated.
In this study, having at least one symptom at the follow-up points was determined as symptom presence. The survey question was as follows: “In the last 7 days, do you still have a symptom that was among your baseline symptoms when you were first diagnosed with COVID-19?” Symptoms were asked one by one in yes/no format and another survey item was available for free-text responses. Data on the symptoms in the acute phase of the disease were collected retrospectively from the patients who were interviewed during the 1st or 3rd month of follow-up. PCR dates, age, gender, and hospital admission were retrieved from the hospital information system. Education, jobs, economic status, smoking, and underlying diseases were patient-reported. Participants were considered fully vaccinated 2 weeks after the second dose of the CoronaVac (inactivated virus) or BNT162b2 (mRNA) vaccine.
Statistical analysis
Categorical variables were summarized as numbers and percentages (n, %). The total number of respondents who completed 1st-, 3rd-, or 6th-month follow-up was used as the denominator for reported symptoms. Percentages of symptom presence at 1st, 3rd, or 6th months were calculated for respondents who reported complaints at the time of diagnosis (clinical infection). Cough and dyspnea/difficulty breathing were categorized as respiratory symptoms. Other symptoms were categorized as mild symptoms. Transitions between no symptom group, respiratory symptom group, and mild symptom group with time were visualized with Sankey plots. Taking account of longitudinal data structure, we fitted generalized estimating equation (GEE) regression models in patients who had baseline symptoms to further evaluate the factors associated with reporting symptoms one, three, and 6 months after diagnosis. We selected a first-order autoregressive AR-1 as the working correlation structure, which allows the correlations of measurements taken farther apart to be less than those taken closer to one another. Model 1 included time and explanatory variables. Model 2 included all explanatory variables. Estimates were presented as odds ratios with 95% confidence intervals. Missing data on baseline explanatory variables of the participants were less than 1.2%, so we made complete case analysis. Data management, analysis, and visualizations were performed with R version 4.0.2 (packages: tidyverse, compareGroups, geepack, sjPlot, ggsankey).
Results
Among a total of 6701 people who tested positive for SARS-CoV-2 RNA, 5610 respondents from two cohorts completed their first interview corresponding either in the 1st month or the 3rd month (response rate for the first interview: 83.7%) (Fig. 1). The total number of dropouts was 1618 (24.1%) for the 3rd month and 2063 (30.8%) for the 6th month. A total of 233 (3.5%) patients have died within 6 months.
In total, 5610 respondents (female 51.8%, age 43.1 ± 15.1) were followed for a mean of 168.3 ± 46.8 days after PCR positivity. Among them, 89.3% (n = 5009) had baseline symptoms (Table 1). The most common symptoms reported were tiredness/fatigue (52.5%), muscle/body aches (52.4%), and loss/change of smell (42.5%). The most common three underlying health conditions were hypertension (15.4%), diabetes (10.5%), and coronary artery disease (6.2%). A total of 8.2% of the patients received inpatient care.
Of the 3727 respondents who completed the 1st-month interview, 37.2% (n = 1387) reported at least one symptom (Table 2). For the 3rd month and 6th months, symptom presence was 21.8% (1108/5083) and 18.2% (844/4638), respectively. Tiredness/fatigue, muscle or body aches, and dyspnea/difficulty breathing were the most common symptoms for the 1st, 3rd, and 6th months of follow-up.
Table 3 shows percentages of reporting symptoms at the 1st, 3rd, and 6th months among the patients who had baseline complaints. Considering the age, symptom presence was most common in the 35–54 years group during the 6 months monitoring. Females reported more symptoms than males (3rd month 28.5 versus 16.8%). Those with bad economical status (45.2%, 26.8%, and 22.8% for time points, respectively) reported more symptoms than those with moderate or good status. There was a positive relationship between the increase in the number of underlying health conditions at baseline, total number of symptoms at baseline, and reporting of symptoms. In asthma patients, symptom presence at the 3rd month was 38.5%, while in patients with chronic renal failure and chronic pulmonary disease, it was 34.2% and 33.8%, respectively. Fully vaccinated people were less likely to report symptoms, especially in the 3rd and the 6th month (9.7 vs. 23.3% and 3.3 vs. 19.5%, respectively).
The transition of symptoms in the patients with initial respiratory symptoms or mild symptoms is illustrated in Fig. 2. For December 2020–May 2021 cohort, 69.6% of the patients with mild symptoms transitioned to no symptom within the first month; while among the patients with respiratory symptoms, 63.3% of the patients transitioned to no symptom within the first month.
The multivariate GEE model indicated that the 35–44 years age group (adjusted OR aOR 1.48, 95% CI 1.22–1.79), 45–54 years group (aOR 1.41, 95% CI 1.16–1.72), and 55–64 years group (aOR 1.34, 95% CI 1.07–1.68) had a higher risk for reporting symptoms compared to 18–24 age group. Female gender (aOR 1.74, 95% CI 1.57–1.93), bad economic status (vs. good economic status) (aOR 1.37, 95% CI 1.14–1.65), current smoking status (vs. non-smokers) (aOR 1.15, 95% CI 1.02–1.29), increasing number of underlying health conditions (≥ 3 conditions, aOR 1.78, 95% CI 1.33–2.37), increasing number of baseline symptoms (> 5 symptoms, aOR 2.47, 95% CI 2.19–2.78), and ICU care (vs. no hospitalization) (aOR 2.18, 95% CI 1.51–3.14) were positively associated with reporting symptoms within 6 months (Table 4). Those fully vaccinated were less likely than unvaccinated individuals to report symptoms (aOR 0.53, 95% CI 0.40–0.72).
Discussion
Using data collected through telephone interviews in DEU-COVIMER, we evaluated the self-reporting of at least one symptom in 6 months with three cross-sectional time frames. We found that 37.2%, 21.8%, and 18.2% of the respondents had at least one symptom for the 1st, 3rd, and 6th months, respectively. At least seven studies investigated the same outcome as in our study: reporting at least one symptom [3,4,5,6,7, 15, 16]. According to these studies, reporting of symptoms preceding ≥ 12 weeks was found to be low as 2.3% [15] or as high as 37.7% [17]. A preprint study combined the results of ten longitudinal study samples and their electronic results in the UK and reported that the percentage of symptoms lasting ≥ 12 weeks was between 7.8 and 17% (Thompson et al., 2021, preprint). Estimates for the post-COVID situations vary widely in the studies because of differences in sample size, different definitions for the outcome, differences in disease severity, and different list of symptoms that were surveyed.
We observed a total of 233 deaths during 6 months of monitoring. Death counts in this study were only based on all-cause mortalities among PCR-positive patients. There is abundant evidence that the risk of developing severe COVID-19 was highly related to old age and comorbidity-specific [18,19,20]. Moreover, the severity of the acute illness had a positive association with reporting of symptoms post-SARS-CoV-2 infection [15], although a study on patients with COVID-19 pneumonia stated otherwise and suggested the biopsychosocial effects of COVID-19 [21]. In our study, female gender, increasing age (except ≥ 75 age group), increase in the number of underlying health conditions, increase in the number of baseline symptoms, and hospitalization were identified as independent risk factors for reporting post-COVID-19 symptoms. These findings were consistent with current literature [15, 21, 22]. Non-association for ≥ 75 age group could be explained by the difficulties in older people expressing their symptoms, misclassification due to information obtained from their relatives, and competing risk of mortality.
Smokers have been found to have a higher risk for COVID-19 progression [23, 24]. A multicenter study from Malaysia found that ever smokers had a higher risk of developing acute respiratory distress syndrome, renal injury, and liver injury [25]. We found an association between reporting symptoms with current smoking. Knowledge of the smoking effect for post-COVID symptoms remains limited in the literature. We could only find one study that indicated persistent symptoms were associated with smoking or vaping [17]. We thought that the respiratory system already damaged by smoking may facilitate severe SARS-CoV-2 infection and, thus, post-COVID symptoms.
The influence of socioeconomic determinants of health on lasting symptoms was not studied widely. We found that perceived bad economical status was associated with increased reporting of symptoms. One preprint study consisting of 1584 patients found that patients with a low perception of socioeconomic status were at greater risk (Thomason et al., 2021, preprint). Additionally, as a more objective indicator, people living in more deprived areas were reported to have a higher burden of persistent symptoms [26]. It seems that disadvantaged people with economic stress and discrimination, as well as those experiencing inequalities in healthcare utilization, were vulnerable populations for lasting symptoms.
The burden of patients experiencing symptoms could overwhelm existing health capacity as the lingering post-COVID effects may cause patients to seek healthcare. It is expected that many of the patients recover spontaneously and there may be no need to investigate a patient with a nonspecific mild clinic if the patient is well. We found that tiredness/fatigue, muscle or body aches, and dyspnea/difficulty breathing were the most common symptoms in all time frames. Patients with rheumatologic disease, chronic renal failure, asthma, and chronic pulmonary disease were more affected patient groups for post-COVID conditions. These risk groups could benefit from planned rehabilitation in conjunction with the clinical decision-making process for differential diagnosis.
During the study period, there were two types of vaccines available in Turkey: CoronaVac and BNT162b2. Vaccination of healthcare workers and older age groups was rolled out on January 14, 2021, with CoronaVac. BNT162b2 was in use as of April 2, 2021. Considering the patient inclusion period from November 1, 2020, to May 31, 2021, we observed 207 vaccine breakthrough infections. We found that being fully vaccinated before having COVID-19 was associated with a decrease in the likelihood of self-reporting symptoms. In a study conducted on mobile phone app users, the odds of having symptoms for 28 days or more after COVID-19 was approximately halved in those who were vaccinated with two doses before infection when compared with unvaccinated controls [27]. This may be due to a reduced risk of developing severe illness among patients with vaccine breakthrough infection [28,29,30,31]. Not every participant in this study had access to vaccines due to the stepwise vaccination strategy. Priority-use groups within the study period were mostly ≥ 55 aged people and healthcare workers. True vaccine effect on post-COVID situations should be evaluated in the studies with more representative of general population.
Our study has several strengths. The study has a large sample size; data from over 5000 patients diagnosed in a public hospital were analyzed. The population-based prospective design increases the generability of our findings while repeated measurements allowed the investigation of changes over time. However, the study has some limitations. Firstly, we had no control group in the study. Although symptom inquiry was conceptualized to COVID-19, the symptoms reported may be due to other respiratory viruses or accompanying diseases themselves. Secondly, DEU-COVIMER survey had 20 items inquiring about symptoms the participants had. Symptoms that were not structured in the questionnaire may have been missed and also may not be declared due to recall bias which is always a limitation in patient-self-report interviews. Thirdly, due to the study period covered, we could not fully evaluate the effect of different SARS-CoV-2 variants. Fourthly, missingness in our data was mostly caused by monotone dropouts which the subjects were fully observed up to a given time but had no monitoring at subsequent times. Dropouts due to mortality, non-responsiveness, and non-participation may lead to biased parameter estimates.
To conclude, this study identifies risk factors for patients who experience post-COVID-19 symptoms. Healthcare providers should appropriately allocate resources prioritizing the patients who would benefit from post-COVID rehabilitation.
Data and/or Code availability
Statistical code is available on request from Ahmet Naci Emecen (ahmetemecen@gmail.com).
References
Callard F, Perego E (2021) How and why patients made Long Covid. Soc Sci Med 268:113426. https://doi.org/10.1016/j.socscimed.2020.113426
Alwan NA, Johnson L (2021) Defining long COVID: going back to the start. Med N Y N 2:501–504. https://doi.org/10.1016/j.medj.2021.03.003
Carfì A, Bernabei R, Landi F et al (2020) Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 324:603–5. https://doi.org/10.1001/jama.2020.12603
Huang C, Huang L, Wang Y et al (2021) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet Elsevier 397:220–232. https://doi.org/10.1016/S0140-6736(20)32656-8
Nehme M, Braillard O, Alcoba G et al (2021) COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med 174:723–725. https://doi.org/10.7326/M20-5926
Petersen MS, Kristiansen MF, Hanusson KD et al (2021) Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis Off Publ Infect Dis Soc Am 73:e4058-63. https://doi.org/10.1093/cid/ciaa1792
Chopra V, Flanders SA, O’Malley M, Malani AN (2021) Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. American College of Physicians. Ann Intern Med 174:576–8. https://doi.org/10.7326/M20-5661
Groff D, Sun A, Ssentongo AE et al (2021) Short-term and long-term rates of postacute sequelae of SARS-CoV-2 ınfection: a systematic review. JAMA Netw Open 4:e2128568. https://doi.org/10.1001/jamanetworkopen.2021.28568
Lopez-Leon S, Wegman-Ostrosky T, Perelman C et al (2021) More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. Nature Publishing Group 11:16144. https://doi.org/10.1038/s41598-021-95565-8
Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V et al (2021) Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an ıntegrative classification. Int J Environ Res Public Health. Multidisciplinary Digital Publishing Institute 18:2621. https://doi.org/10.3390/ijerph18052621
Tenforde MW, Kim SS, Lindsell CJ et al (2020) Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep 69:993–8. https://doi.org/10.15585/mmwr.mm6930e1
Soriano JB, Murthy S, Marshall JC et al (2021) A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis S1473309921007039. https://doi.org/10.1016/S1473-3099(21)00703-9
Sigfrid L, Cevik M, Jesudason E et al (2021) What is the recovery rate and risk of long-term consequences following a diagnosis of COVID-19? A harmonised, global longitudinal observational study protocol. BMJ Open 11:e043887. https://doi.org/10.1136/bmjopen-2020-043887
Evans RA, McAuley H, Harrison EM et al (2021) Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 9:1275–1287. https://doi.org/10.1016/S2213-2600(21)00383-0
Sudre CH, Murray B, Varsavsky T et al (2021) Attributes and predictors of long COVID. Nat Med Nature Publishing Group 27:626–631. https://doi.org/10.1038/s41591-021-01292-y
Klein H, Asseo K, Karni N et al (2021) Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis S1198–743X(21)00083–5. https://doi.org/10.1016/j.cmi.2021.02.008
Whitaker M, Elliott J, Chadeau-Hyam M et al (2022) Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat Commun 13(1):1957. https://doi.org/10.1038/s41467-022-29521-z
Williamson EJ, Walker AJ, Bhaskaran K et al (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature Nature Publishing Group 584:430–436. https://doi.org/10.1038/s41586-020-2521-4
Dessie ZG, Zewotir T (2021) Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis 21:855. https://doi.org/10.1186/s12879-021-06536-3
Booth A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D et al (2021) Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE. Public Library of Science 16:e0247461. https://doi.org/10.1371/journal.pone.0247461
Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG (2021) Post-COVID-19 symptom burden: what is long-COVID and how should we manage ıt? Lung 199:113–119. https://doi.org/10.1007/s00408-021-00423-z
Mahmud R, Rahman MM, Rassel MA, Monayem FB, Sayeed SKJB, Islam MS et al (2021) Post-COVID-19 syndrome among symptomatic COVID-19 patients: A prospective cohort study in a tertiary care center of Bangladesh. PLoS ONE. Public Library of Science 16:e0249644. https://doi.org/10.1371/journal.pone.0249644
Kokturk N, Babayigit C, Kul S et al (2021) The predictors of COVID-19 mortality in a nationwide cohort of Turkish patients. Respir Med 183:106433. https://doi.org/10.1016/j.rmed.2021.106433
Gülsen A, Yigitbas BA, Uslu B et al (2020) The effect of smoking on COVID-19 Symptom severity: systematic review and meta-analysis. Pulm Med e7590207. https://doi.org/10.1155/2020/7590207
Ismail N, Hassan N, Abd Hamid MHN et al (2022) Association of smoking and severity of COVID-19 infection among 5,889 patients in Malaysia: a multi-center observational study. Int J Infect Dis 116:189–196. https://doi.org/10.1016/j.ijid.2022.01.011
Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK - Office for National Statistics (2022) https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/3march2022. Assessed: 06 April 2022
Antonelli M, Penfold RS, Merino J et al (2022) Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis Elsevier 22:43–55. https://doi.org/10.1016/S1473-3099(21)00460-6
Tenforde MW, Self WH, Adams K et al (2021) Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA 326:2043–2054. https://doi.org/10.1001/jama.2021.19499
Agrawal U, Katikireddi SV, McCowan C et al (2021) COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2·57 million people in Scotland (EAVE II): a prospective cohort study. Lancet Respir Med Elsevier 9:1439–1449. https://doi.org/10.1016/S2213-2600(21)00380-5
Bahl A, Johnson S, Maine G et al (2021) Vaccination reduces need for emergency care in breakthrough COVID-19 infections: a multicenter cohort study. Lancet Reg Health Am 4:100065. https://doi.org/10.1016/j.lana.2021.100065
Butt AA, Nafady-Hego H, Chemaitelly H et al (2021) Outcomes among patients with breakthrough SARS-CoV-2 ınfection after vaccination. Int J Infect Dis Elsevier 110:353–358. https://doi.org/10.1016/j.ijid.2021.08.008
Acknowledgements
We thank all DEU-COVIMER staff and all of the participating individuals in the study.
Author information
Authors and Affiliations
Contributions
BU and ANE were responsible for the conceptualization and supervision of the study. ANE, BU, FD, OK, VAO, and SB designed the methodology. SK, OT, AFS, NS, and EBS were responsible for data curation. ANE, BU, SK, and OT made statistical analyses. ANE, BU, OK, and VAO were involved in writing the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the ethics committee of Dokuz Eylul University (No: 2021/02–66). The procedures used in this study adhere to tenets of the Declaration of Helsinki.
Consent to participate
Verbal informed consent was obtained prior to the interview.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Emecen, A.N., Keskin, S., Turunc, O. et al. The presence of symptoms within 6 months after COVID-19: a single-center longitudinal study. Ir J Med Sci 192, 741–750 (2023). https://doi.org/10.1007/s11845-022-03072-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-03072-0