Abstract
Background
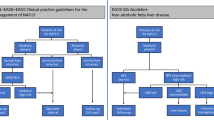
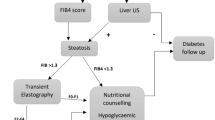
Fatty liver disease and fibrosis are common in patients with type 2 diabetes mellitus (T2DM). Recently published European Association for the Study of the Liver guidelines have suggested screening such patients using liver stiffness measurement (LSM) or fibrosis-4 index (FIB-4) to exclude advanced fibrosis.
Aims
We initiated a screening programme at the diabetes out-patient clinic to assess the reliability of the suggested approaches and resulting referrals.
Methods
In this prospective study, consecutive patients attending for T2DM review at an Irish level 3 (district general) hospital between September and November 2021 were screened for liver fibrosis using LSM and had their FIB-4 calculated. The first 100 patients with valid LSM measurements were included in the analysis.
Results
Referral rates to the hepatology clinic varied by modality used. If FIB-4 ≥ 1.3 criterion was used, the referral rate to the hepatology clinic was 45%; using LSM < 8 kPa to rule out advanced fibrosis resulted in 34% referral rate; using LSM ≥ 10 kPa to suggest probable compensated advanced chronic liver disease reduced referral rates to 15%. Combining FIB-4 with LSM in a two-step algorithm led to missed potentially significant liver disease in large numbers. 47% patients with LSM ≥ 8 kPa and 33% with LSM ≥ 10 kPa had FIB-4 < 1.3.
Conclusions
Screening of patients with T2DM using LSM alone rather than FIB-4 leads to reduced numbers of, and more appropriate, referrals to the hepatology clinic. Shifting from an exclusion (LSM < 8 kPa) to an inclusion based (LSM ≥ 10 kPa) approach may lessen the potential of screening to overwhelm hepatology services.

Similar content being viewed by others
References
Younossi ZM, Golabi P, de Avila L et al (2019) The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 71:793–801. https://doi.org/10.1016/j.jhep.2019.06.021
Targher G, Corey KE, Byrne CD, Roden M (2021) The complex link between NAFLD and type 2 diabetes mellitus — mechanisms and treatments. Nat Rev Gastroenterol Hepatol 18:599–612
Alexopoulos AS, Duffy R, Kobe EA et al (2021) Underrecognition of nonalcoholic fatty liver disease in poorly controlled diabetes: a call to action in diabetes care. J Endocr Soc 5:1–8. https://doi.org/10.1210/jendso/bvab155
Association AD (2021) Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes−2021. Diabetes Care 44:S40–S52. https://doi.org/10.2337/dc21-S004
Chalasani N, Younossi Z, Lavine JE et al (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67:328–357. https://doi.org/10.1002/hep.29367
Berzigotti A, Tsochatzis E, Boursier J et al (2021) EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol 75:659–689. https://doi.org/10.1016/j.jhep.2021.05.025
Boursier J, Zarski JP, de Ledinghen V et al (2013) Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 57:1182–1191. https://doi.org/10.1002/hep.25993
Sterling RK, Lissen E, Clumeck N et al (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43:1317–1325. https://doi.org/10.1002/hep.21178
Eslam M, Newsome PN, Sarin SK et al (2020) A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 73:202–209
Wai-Sun Wong V, Kanwal F (2021) On the proposed definition of metabolic-associated fatty liver disease. Clin Gastroenterol Hepatol 19:865–870
Mózes FE, Lee JA, Selvaraj EA et al (2021) Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut. https://doi.org/10.1136/gutjnl-2021-324243
Bril F, McPhaul MJ, Caulfield MP et al (2020) Performance of plasma biomarkers and diagnostic panels for nonalcoholic steatohepatitis and advanced fibrosis in patients with type 2 diabetes. Diabetes Care 43:290–297. https://doi.org/10.2337/dc19-1071
Ciardullo S, Monti T, Perseghin G (2021) High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care 44:519–525. https://doi.org/10.2337/dc20-1778
Lomonaco R, Leiva EG, Bril F et al (2021) Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care 44:399–406. https://doi.org/10.2337/dc20-1997
Bertot LC, Jeffrey GP, de Boer B et al (2018) Diabetes impacts prediction of cirrhosis and prognosis by non-invasive fibrosis models in non-alcoholic fatty liver disease. Liver Int 38:1793–1802. https://doi.org/10.1111/liv.13739
Tovo CV, Villela-Nogueira CA, Leite NC et al (2019) Transient hepatic elastography has the best performance to evaluate liver fibrosis in non-alcoholic fatty liver disease (NAFLD). Ann Hepatol 18:445–449. https://doi.org/10.1016/j.aohep.2018.09.003
Marchesini G, Day CP, Dufour JF et al (2016) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64:1388–1402. https://doi.org/10.1016/j.jhep.2015.11.004
Alexopoulos AS, Crowley MJ, Wang Y et al (2021) Glycemic control predicts severity of hepatocyte ballooning and hepatic fibrosis in nonalcoholic fatty liver disease. Hepatology 74:1220–1233. https://doi.org/10.1002/hep.31806
Davies MJ, D’Alessio DA, Fradkin J et al (2018) Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 61:2461–2498. https://doi.org/10.1007/s00125-018-4729-5
Newsome PN, Buchholtz K, Cusi K et al (2021) A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 384:1113–1124. https://doi.org/10.1056/nejmoa2028395
The Emerging Risk Factors Collaboration (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364:829–841. https://doi.org/10.1056/nejmoa1008862
Angeli P, Bernardi M, Villanueva C et al (2018) EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 69:406–460. https://doi.org/10.1016/j.jhep.2018.03.024
Shanahan W, Jacob B, McCarthy C et al (2022) An exploratory analysis of patient factors influencing acceptance of extended criteria liver grafts. Ann Hepatol 27:100686. https://doi.org/10.1016/j.aohep.2022.100686
de Franchis R, Faculty BVI (2015) Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 63:743–752. https://doi.org/10.1016/J.JHEP.2015.05.022
Chuah KH, Lai LL, Vethakkan SR et al (2020) Liver stiffness measurement in non-alcoholic fatty liver disease: two is better than one. J Gastroenterol Hepatol 35:1404–1411. https://doi.org/10.1111/jgh.14978
Chow JCL, Wong GLH, Chan AWH et al (2019) Repeating measurements by transient elastography in non-alcoholic fatty liver disease patients with high liver stiffness. J Gastroenterol Hepatol 34:241–248. https://doi.org/10.1111/jgh.14311
Lee HW, Wong GLH, Kwok R et al (2020) Serial transient elastography examinations to monitor patients with type 2 diabetes: a prospective cohort study. Hepatology 72:1230–1241. https://doi.org/10.1002/hep.31142
Nogami A, Yoneda M, Kobayashi T et al (2019) Assessment of 10-year changes in liver stiffness using vibration-controlled transient elastography in non-alcoholic fatty liver disease. Hepatol Res 49:872–880. https://doi.org/10.1111/hepr.13349
O’Gorman P, Naimimohasses S, Monaghan A et al (2020) Improvement in histological endpoints of MAFLD following a 12-week aerobic exercise intervention. Aliment Pharmacol Ther 52:1387–1398. https://doi.org/10.1111/apt.15989
Liu SYW, Wong VWS, Wong SKH et al (2021) A prospective 5-year study on the use of transient elastography to monitor the improvement of non-alcoholic fatty liver disease following bariatric surgery. Sci Rep 11:1–11. https://doi.org/10.1038/s41598-021-83782-0
Kwok R, Choi KC, Wong GLH et al (2016) Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 65:1359–1368. https://doi.org/10.1136/gutjnl-2015-309265
Lai LL, Wan Yusoff WNI, Vethakkan SR et al (2019) Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol 34:1396–1403. https://doi.org/10.1111/JGH.14577
Roulot D, Roudot-Thoraval F, NKontchou G et al (2017) Concomitant screening for liver fibrosis and steatosis in French type 2 diabetic patients using fibroscan. Liver Int 37:1897–1906. https://doi.org/10.1111/liv.13481
Sporea I, Mare R, Popescu A et al (2020) Screening for liver fibrosis and steatosis in a large cohort of patients with type 2 diabetes using vibration controlled transient elastography and controlled attenuation parameter in a single-center real-life experience. J Clin Med. https://doi.org/10.3390/jcm9041032
Mantovani A, Turino T, Lando MG et al (2020) Screening for non-alcoholic fatty liver disease using liver stiffness measurement and its association with chronic kidney disease and cardiovascular complications in patients with type 2 diabetes. Diabetes Metab 46:296–303. https://doi.org/10.1016/j.diabet.2019.11.004
Tuong TTK, Tran DK, Phu PQT et al (2020) Non-alcoholic fatty liver disease in patients with type 2 diabetes: evaluation of hepatic fibrosis and steatosis using fibroscan. Diagnostics. https://doi.org/10.3390/diagnostics10030159
Chen K, Sng WK, Quah JHM et al (2020) Clinical spectrum of non-alcoholic fatty liver disease in patients with diabetes mellitus. PLoS ONE 15:e0236977. https://doi.org/10.1371/journal.pone.0236977
Ratchatasettakul K, Rattanasiri S, Promson K et al (2017) The inverse effect of meal intake on controlled attenuation parameter and liver stiffness as assessed by transient elastography. BMC Gastroenterol 17:1–7. https://doi.org/10.1186/s12876-017-0609-6
Author information
Authors and Affiliations
Contributions
Conceptualization: William Shanahan, Mary Jane Brassill, Paud O’Regan; methodology: William Shanahan, Isha Bagwe; formal analysis and investigation: William Shanahan, Isha Bagwe; writing — original draft preparation: William Shanahan; writing — review and editing: Isha Bagwe, Mary Jane Brassill, Paud O’Regan; supervision: Mary Jane Brassill, Paud O’Regan.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shanahan, W., Bagwe, I., Brassill, M. et al. Reduced and more appropriate referrals of patients with type 2 diabetes using liver stiffness measurement compared to FIB-4. Ir J Med Sci 192, 649–654 (2023). https://doi.org/10.1007/s11845-022-03019-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-03019-5