Abstract
Introduction
Mutations/variants in mitochondrial genomes are found to be associated with type 2 diabetes mellitus (T2DM), but the pathophysiology of this disease remains largely unknown.
Aim
The aim of this study is to investigate the relationship between mitochondrial DNA (mtDNA) variants and T2DM.
Methodology
A maternally inherited T2DM pedigree is underwent clinical, genetic, and molecular assessment. Moreover, the complete mitochondrial genomes of the matrilineal relatives of this family are PCR amplified and sequenced. We also utilize the phylogenetic conservation analysis, haplogroup classification, and the pathogenicity scoring system to determine the T2DM-associated potential pathogenic mtDNA variants.
Result
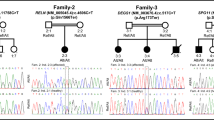
Four of seven matrilineal relatives of this pedigree suffered from T2DM with variable ages of onset. Screening for the entire mtDNA genes of matrilineal members reveals co-existence of ND5 T12338C and tRNAAla T5587C variants, as well as 21 genetic polymorphisms which belong to East Asian haplogroup F2. Interestingly, the T12338C variant causes the alternation of first amino acid Met to Thr, shortened two amino acids of ND5 protein. Furthermore, T5587C variant is located at position 73 in the 3’end of mt-tRNAAla and may have structural and functional consequences.
Conclusions
The co-occurrence of ND5 T12338C and tRNAAla T5587C variants may impair the mitochondrial function, which are associated with the development of T2DM in this family.




Similar content being viewed by others
References
Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414(6865):782–787
Roglic G, Unwin N (2010) Mortality attributable to diabetes: estimates for the year 2010. Diabetes Res Clin Pract 87(1):15–19
Festa A, Williams K, D’Agostino R Jr et al (2006) The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes 55(4):1114–1120
Kumar P, Liu C, Suliburk JW et al (2020) Supplementing glycine and N-acetylcysteine (GlyNAC) in aging HIV patients improves oxidative stress, mitochondrial dysfunction, inflammation, endothelial dysfunction, insulin resistance, genotoxicity, strength, and cognition: results of an open-label clinical trial. Biomedicines 8(10):E390
Townsend LK, Brunetta BS, Mori MA (2020) Mitochondria-associated ER membranes in glucose homeostasis and insulin resistance. Am J Physiol Endocrinol Metab 319(6):E1053–E1060
Pinti MV, Fink GK, Hathaway QA et al (2019) Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. Am J Physiol Endocrinol Metab 316(2):E268–E285
Skuratovskaia D, Komar A, Vulf M et al (2020) Mitochondrial destiny in type 2 diabetes: the effects of oxidative stress on the dynamics and biogenesis of mitochondria. PeerJ 8: e9741
He ZF, Zheng LC, Xie DY et al (2017) Mutational analysis of mitochondrial tRNA genes in patients with lung cancer. Balkan J Med Genet 19(2):45–50
Guo ZS, Jin CL, Yao ZJ et al (2017) Analysis of the mitochondrial 4977 Bp deletion in patients with hepatocellular carcinoma. Balkan J Med Genet 20(1):81–86
Majamaa K, Moilanen JS, Uimonen S et al (1998) Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: prevalence of the mutation in an adult population. Am J Hum Genet 63(2):447–454
Ding Y, Xia BH, Zhang CJ et al (2018) Mitochondrial tRNALeu(UUR) C3275T, tRNAGln T4363C and tRNALys A8343G mutations may be associated with PCOS and metabolic syndrome. Gene 642:299–306
American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl. 1):S62–S69
Lin L, Cui P, Qiu Z et al (2019) The mitochondrial tRNAAla 5587T>C and tRNALeu(CUN) 12280A>G mutations may be associated with hypertension in a Chinese family. Exp Ther Med 17(3):1855–1862
Committee JN, on Prevention, Detection, Evaluation and Treatment of High Blood Pressure, (1997) The sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Arch Intern Med 157(21):2413–2446
Qu J, Li R, Zhou X et al (2006) The novel A4435G mutation in the mitochondrial tRNAMet may modulate the phenotypic expression of the LHON-associated ND4 G11778A mutation. Invest Ophthalmol Vis Sci 47(2):475–483
Ding Y, Zhuo G, Zhang C (2016) The mitochondrial tRNALeu(UUR) A3302G mutation may be associated with insulin resistance in woman with polycystic ovary syndrome. Reprod Sci 23(2):228–233
Rieder MJ, Taylor SL, Tobe VO et al (1998) Automating the identification of DNA variations using quality-based fluorescence re-sequencing: analysis of the human mitochondrial genome. Nucleic Acids Res 26(4):967–973
Andrews RM, Kubacka I, Chinnery PF et al (1999) Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 23(2):147
Levin L, Zhidkov I, Gurman Y et al (2013) Functional recurrent mutations in the human mitochondrial phylogeny: dual roles in evolution and disease. Genome Biol Evol 5(5):876–890
Kong QP, Bandelt HJ, Sun C et al (2006) Updating the East Asian mtDNA phylogeny: a prerequisite for the identification of pathogenic mutations. Hum Mol Genet 15(13):2076–2086
Yarham JW, Al-Dosary M, Blakely EL et al (2011) A comparative analysis approach to determining the pathogenicity of mitochondrial tRNA mutations. Hum Mutat 32(11):1319–1325
López-Lluch G, Hernández-Camacho JD, Fernández-Ayala DJM et al (2018) Mitochondrial dysfunction in metabolism and ageing: shared mechanisms and outcomes? Biogerontology 19(6):461–480
Bibb MJ, Van Etten RA, Wright CT et al (1981) Sequence and gene organization of mouse mitochondrial DNA. Cell 26(2 Pt 2):167–180
Gadaleta G, Pepe G, De Candia G et al (1989) The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol 28(6):497–516
Roe BA, Ma DP, Wilson RK et al (1985) The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem 260(17):9759–9774
Ji Y, Qiao L, Liang X et al (2017) Leber's hereditary optic neuropathy is potentially associated with a novel m.5587T>C mutation in two pedigrees. Mol Med Rep 16(6): 8997–9004
Jiang P, Ling Y, Zhu T et al (2020) Mitochondrial tRNA mutations in Chinese children with tic disorders. Biosci Rep 40(12): BSR20201856
Tang X, Li R, Zheng J et al (2010) Maternally inherited hearing loss is associated with the novel mitochondrial tRNA Ser (UCN) 7505T>C mutation in a Han Chinese family. Mol Genet Metab 100(1):57–64
Crimi M, Sciacco M, Galbiati S et al (2002) A collection of 33 novel human mtDNA homoplasmic variants. Hum Mutat 20(5):409
Ji Y, Nie Z, Meng F et al (2021) Mechanistic insights into mitochondrial tRNAAla 3'-end metabolism deficiency. J Biol Chem 297(1): 100816.
Liu XL, Zhou X, Zhou J et al (2011) Leber’s hereditary optic neuropathy is associated with the T12338C mutation in mitochondrial ND5 gene in six Han Chinese families. Ophthalmology 118(5):978–985
Ding Y, Zhuo G, Zhang C et al (2016) Point mutation in mitochondrial tRNA gene is associated with polycystic ovary syndrome and insulin resistance. Mol Med Rep 13(4):3169–3172
Zhang J, Ji Y, Lu Y et al (2018) Leber’s hereditary optic neuropathy (LHON)-associated ND5 12338T > C mutation altered the assembly and function of complex I, apoptosis and mitophagy. Hum Mol Genet 27(11):1999–2011
Teng L, Zheng J, Leng J et al (2012) Clinical and molecular characterization of a Han Chinese family with high penetrance of essential hypertension. Mitochondrial DNA 23(6):461–465
Chen B, Sun D, Yang L et al (2008) Mitochondrial ND5 T12338C, tRNA(Cys) T5802C, and tRNA(Thr) G15927A variants may have a modifying role in the phenotypic manifestation of deafness-associated 12S rRNA A1555G mutation in three Han Chinese pedigrees. Am J Med Genet A 146A(10):1248–1258
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This work is approved by the Ethics Committee of Second Affiliated Hospital of Zhejiang University (Approval Number: 2021–0382).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, Z., Cai, X., Kong, J. et al. Maternally transmitted diabetes mellitus may be associated with mitochondrial ND5 T12338C and tRNAAla T5587C variants. Ir J Med Sci 191, 2625–2633 (2022). https://doi.org/10.1007/s11845-021-02911-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-021-02911-w