Abstract
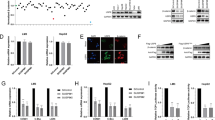
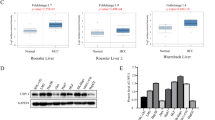
Hepatocellular carcinoma (HCC) is among the malignant tumors with highest mortality. The role of USP9X in the carcinogenesis of HCC has not yet been determined. In this study, USP9X was found significantly highly expressed in the intratumor tissues. Expression of intratumor USP9X was associated with tumor size and microvascular invasion while USP9X is independent risk factor of HCC disease-free survival and overall survival. In vitro studies revealed that knockdown of USP9X significantly inhibited the proliferation of HCC cells. Mechanically, USP9X promotes HCC cell proliferation by regulating the expression of beta-catenin. The results of the present study demonstrated that high expression of USP9X in intratumoral cells is associated with poor HCC prognosis, which may serve as a potential target for an adjuvant therapy.




Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- USP9X:
-
Ubiquitin-specific protease 9, X chromosome
- HBV/HCV:
-
Hepatitis B/C virus
- NASH:
-
Non-alcoholic steatohepatitis
- TGF:
-
Transforming growth factor
- MAPK:
-
Mitogen-activated protein kinase
- ERK:
-
Extracellular regulated protein kinases
- ASK1:
-
Apoptosis signal-regulating kinase 1
- TCF/LEF:
-
T-cell factor/lymphoid enhancer-binding factor
- AFP:
-
α-Fetoprotein
References
Jiang Y, Sun A, Zhao Y, Ying W, Sun H, Yang X, Xing B, Sun W, Ren L, Hu B, Li C, Zhang L, Qin G, Zhang M, Chen N, Zhang M, Huang Y, Zhou J, Zhao Y, Liu M, Zhu X, Qiu Y, Sun Y, Huang C, Yan M, Wang M, Liu W, Tian F, Xu H, Zhou J, Wu Z, Shi T, Zhu W, Qin J, Xie L, Fan J, Qian X, He F, Chinese Human Proteome Project C (2019) Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 567(7747):257–261. https://doi.org/10.1038/s41586-019-0987-8
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
The L (2018) GLOBOCAN 2018: counting the toll of cancer. Lancet 392(10152):985. https://doi.org/10.1016/S0140-6736(18)32252-9
Arzumanyan A, Reis HM, Feitelson MA (2013) Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer 13(2):123–135. https://doi.org/10.1038/nrc3449
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet 391(10127):1301–1314. https://doi.org/10.1016/S0140-6736(18)30010-2
Gao Q, Zhu H, Dong L, Shi W, Chen R, Song Z, Huang C, Li J, Dong X, Zhou Y, Liu Q, Ma L, Wang X, Zhou J, Liu Y, Boja E, Robles AI, Ma W, Wang P, Li Y, Ding L, Wen B, Zhang B, Rodriguez H, Gao D, Zhou H, Fan J (2019) Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell 179(2):561–577 e522. https://doi.org/10.1016/j.cell.2019.08.052
Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, Han M, Thornton AM, Zhang H, Egger M, Luo J, Felsher DW, McVicar DW, Weber A, Heikenwalder M, Greten TF (2016) NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531(7593):253–257. https://doi.org/10.1038/nature16969
Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G, Investigators R (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389(10064):56–66. https://doi.org/10.1016/S0140-6736(16)32453-9
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391(10126):1163–1173. https://doi.org/10.1016/S0140-6736(18)30207-1
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088):2492–2502. https://doi.org/10.1016/S0140-6736(17)31046-2
Perez-Mancera PA, Rust AG, van der Weyden L, Kristiansen G, Li A, Sarver AL, Silverstein KA, Grutzmann R, Aust D, Rummele P, Knosel T, Herd C, Stemple DL, Kettleborough R, Brosnan JA, Li A, Morgan R, Knight S, Yu J, Stegeman S, Collier LS, ten Hoeve JJ, de Ridder J, Klein AP, Goggins M, Hruban RH, Chang DK, Biankin AV, Grimmond SM, Australian Pancreatic Cancer Genome I, Wessels LF, Wood SA, Iacobuzio-Donahue CA, Pilarsky C, Largaespada DA, Adams DJ, Tuveson DA (2012) The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature 486(7402):266–270. https://doi.org/10.1038/nature11114
Wood SA, Pascoe WS, Ru K, Yamada T, Hirchenhain J, Kemler R, Mattick JS (1997) Cloning and expression analysis of a novel mouse gene with sequence similarity to the Drosophila fat facets gene. Mech Dev 63(1):29–38. https://doi.org/10.1016/s0925-4773(97)00672-2
Dupont S, Mamidi A, Cordenonsi M, Montagner M, Zacchigna L, Adorno M, Martello G, Stinchfield MJ, Soligo S, Morsut L, Inui M, Moro S, Modena N, Argenton F, Newfeld SJ, Piccolo S (2009) FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell 136(1):123–135. https://doi.org/10.1016/j.cell.2008.10.051
Zhang J, Wang J, Luan T, Zuo Y, Chen J, Zhang H, Ye Z, Wang H, Hai B (2019) Deubiquitinase USP9X regulates the invasion of prostate cancer cells by regulating the ERK pathway and mitochondrial dynamics. Oncol Rep 41(6):3292–3304. https://doi.org/10.3892/or.2019.7131
Nagai H, Noguchi T, Homma K, Katagiri K, Takeda K, Matsuzawa A, Ichijo H (2009) Ubiquitin-like sequence in ASK1 plays critical roles in the recognition and stabilization by USP9X and oxidative stress-induced cell death. Mol Cell 36(5):805–818. https://doi.org/10.1016/j.molcel.2009.10.016
Jingjing W, Wenzheng G, Donghua W, Guangyu H, Aiping Z, Wenjuan W (2018) Deubiquitination and stabilization of programmed cell death ligand 1 by ubiquitin-specific peptidase 9, X-linked in oral squamous cell carcinoma. Cancer Med 7(8):4004–4011. https://doi.org/10.1002/cam4.1675
Liu L, Yao D, Zhang P, Ding W, Zhang X, Zhang C, Gong S, Zhang Y, Wang J, Sun T, Ren Z (2017) Deubiquitinase USP9X promotes cell migration, invasion and inhibits apoptosis of human pancreatic cancer. Oncol Rep 38(6):3531–3537. https://doi.org/10.3892/or.2017.6050
Ruiz de Galarreta M, Bresnahan E, Molina-Sanchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V, Casanova-Acebes M, Dhainaut M, Villacorta-Martin C, Singhi AD, Moghe A, von Felden J, Tal Grinspan L, Wang S, Kamphorst AO, Monga SP, Brown BD, Villanueva A, Llovet JM, Merad M, Lujambio A (2019) Beta-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov 9(8):1124–1141. https://doi.org/10.1158/2159-8290.CD-19-0074
Shang N, Wang H, Bank T, Perera A, Joyce C, Kuffel G, Zilliox MJ, Cotler SJ, Ding X, Dhanarajan A, Breslin P, Qiu W (2019) Focal adhesion kinase and beta-catenin cooperate to induce hepatocellular carcinoma. Hepatology. https://doi.org/10.1002/hep.30707
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281(5382):1509–1512. https://doi.org/10.1126/science.281.5382.1509
Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398(6726):422–426. https://doi.org/10.1038/18884
Yang B, Zhang S, Wang Z, Yang C, Ouyang W, Zhou F, Zhou Y, Xie C (2016) Deubiquitinase USP9X deubiquitinates beta-catenin and promotes high grade glioma cell growth. Oncotarget 7(48):79515–79525. https://doi.org/10.18632/oncotarget.12819
Sun R, Liu Z, Qiu B, Chen T, Li Z, Zhang X, Xu Y, Zhang Z (2019) Annexin10 promotes extrahepatic cholangiocarcinoma metastasis by facilitating EMT via PLA2G4A/PGE2/STAT3 pathway. EBioMedicine 47:142–155. https://doi.org/10.1016/j.ebiom.2019.08.062
Wang HM, Xu YF, Ning SL, Yang DX, Li Y, Du YJ, Yang F, Zhang Y, Liang N, Yao W, Zhang LL, Gu LC, Gao CJ, Pang Q, Chen YX, Xiao KH, Ma R, Yu X, Sun JP (2014) The catalytic region and PEST domain of PTPN18 distinctly regulate the HER2 phosphorylation and ubiquitination barcodes. Cell Res 24(9):1067–1090. https://doi.org/10.1038/cr.2014.99
Liu H, Xu Y, Zhang Q, Li K, Wang D, Li S, Ning S, Yang H, Shi W, Liu Z, Chen Y (2017) Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep 7:44275. https://doi.org/10.1038/srep44275
Xu YF, Yang XQ, Lu XF, Guo S, Liu Y, Iqbal M, Ning SL, Yang H, Suo N, Chen YX (2014) Fibroblast growth factor receptor 4 promotes progression and correlates to poor prognosis in cholangiocarcinoma. Biochem Biophys Res Commun 446(1):54–60. https://doi.org/10.1016/j.bbrc.2014.02.050
Xu YF, Ge FJ, Han B, Yang XQ, Su H, Zhao AC, Zhao MH, Yang YB, Yang J (2015) High-mobility group box 1 expression and lymph node metastasis in intrahepatic cholangiocarcinoma. World J Gastroenterol 21(11):3256–3265. https://doi.org/10.3748/wjg.v21.i11.3256
Xu YF, Liu ZL, Pan C, Yang XQ, Ning SL, Liu HD, Guo S, Yu JM, Zhang ZL (2019) HMGB1 correlates with angiogenesis and poor prognosis of perihilar cholangiocarcinoma via elevating VEGFR2 of vessel endothelium. Oncogene 38(6):868–880. https://doi.org/10.1038/s41388-018-0485-8
Liu H, Xu Y, Zhang Q, Yang H, Shi W, Liu Z, Li K, Gong Z, Ning S, Li S, Chen Y (2017) Prognostic significance of TBL1XR1 in predicting liver metastasis for early stage colorectal cancer. Surg Oncol 26(1):13–20. https://doi.org/10.1016/j.suronc.2016.12.003
Xu Y, Yang X, Li Z, Li S, Guo S, Ismail S, Liu H, Huang Z, Zhang Z, Chen Y, Sun Q (2017) Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget 8(3):4888–4900. https://doi.org/10.18632/oncotarget.13982
Xu YF, Liu HD, Liu ZL, Pan C, Yang XQ, Ning SL, Zhang ZL, Guo S, Yu JM (2018) Sprouty2 suppresses progression and correlates to favourable prognosis of intrahepatic cholangiocarcinoma via antagonizing FGFR2 signalling. J Cell Mol Med 22(11):5596–5606. https://doi.org/10.1111/jcmm.13833
Liu Z, Sun R, Zhang X, Qiu B, Chen T, Li Z, Xu Y, Zhang Z (2019) Transcription factor 7 promotes the progression of perihilar cholangiocarcinoma by inducing the transcription of c-Myc and FOS-like antigen 1. EBioMedicine 45:181–191. https://doi.org/10.1016/j.ebiom.2019.06.023
Meng X, Liu X, Guo X, Jiang S, Chen T, Hu Z, Liu H, Bai Y, Xue M, Hu R, Sun SC, Liu X, Zhou P, Huang X, Wei L, Yang W, Xu C (2018) FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature 564(7734):130–135. https://doi.org/10.1038/s41586-018-0756-0
Yang Y, Staudt LM (2015) Protein ubiquitination in lymphoid malignancies. Immunol Rev 263(1):240–256. https://doi.org/10.1111/imr.12247
Williams SA, Maecker HL, French DM, Liu J, Gregg A, Silverstein LB, Cao TC, Carano RA, Dixit VM (2011) USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell 146(6):918–930. https://doi.org/10.1016/j.cell.2011.07.040
Cai JB, Shi GM, Dong ZR, Ke AW, Ma HH, Gao Q, Shen ZZ, Huang XY, Chen H, Yu DD, Liu LX, Zhang PF, Zhang C, Hu MY, Yang LX, Shi YH, Wang XY, Ding ZB, Qiu SJ, Sun HC, Zhou J, Shi YG, Fan J (2015) Ubiquitin-specific protease 7 accelerates p14(ARF) degradation by deubiquitinating thyroid hormone receptor-interacting protein 12 and promotes hepatocellular carcinoma progression. Hepatology 61(5):1603–1614. https://doi.org/10.1002/hep.27682
Bridges CR, Tan MC, Premarathne S, Nanayakkara D, Bellette B, Zencak D, Domingo D, Gecz J, Murtaza M, Jolly LA, Wood SA (2017) USP9X deubiquitylating enzyme maintains RAPTOR protein levels, mTORC1 signalling and proliferation in neural progenitors. Sci Rep 7(1):391. https://doi.org/10.1038/s41598-017-00149-0
Wolfsperger F, Hogh-Binder SA, Schittenhelm J, Psaras T, Ritter V, Bornes L, Huber SM, Jendrossek V, Rudner J (2016) Deubiquitylating enzyme USP9x regulates radiosensitivity in glioblastoma cells by Mcl-1-dependent and -independent mechanisms. Cell Death Dis 7:e2039. https://doi.org/10.1038/cddis.2015.405
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Linyi people’s Hospital and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, My., Li, Zp., Sun, Zn. et al. USP9X promotes the progression of hepatocellular carcinoma by regulating beta-catenin. Ir J Med Sci 189, 865–871 (2020). https://doi.org/10.1007/s11845-020-02199-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-020-02199-2